Protein and RNA dynamical fingerprinting
- PMID: 30833555
- PMCID: PMC6399446
- DOI: 10.1038/s41467-019-08926-3
Protein and RNA dynamical fingerprinting
Abstract
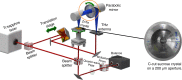
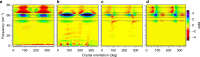
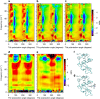
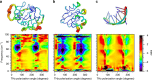
Protein structural vibrations impact biology by steering the structure to functional intermediate states; enhancing tunneling events; and optimizing energy transfer. Strong water absorption and a broad continuous vibrational density of states have prevented optical identification of these vibrations. Recently spectroscopic signatures that change with functional state were measured using anisotropic terahertz microscopy. The technique however has complex sample positioning requirements and long measurement times, limiting access for the biomolecular community. Here we demonstrate that a simplified system increases spectroscopic structure to dynamically fingerprint biomacromolecules with a factor of 6 reduction in data acquisition time. Using this technique, polarization varying anisotropy terahertz microscopy, we show sensitivity to inhibitor binding and unique vibrational spectra for several proteins and an RNA G-quadruplex. The technique's sensitivity to anisotropic absorbance and birefringence provides rapid assessment of macromolecular dynamics that impact biology.
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Polarization-varying anisotropic terahertz microscopy.Nat Methods. 2019 May;16(5):362. doi: 10.1038/s41592-019-0419-6. Nat Methods. 2019. PMID: 31040431 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

