In vivo investigation of hyperpolarized [1,3-13C2]acetoacetate as a metabolic probe in normal brain and in glioma
- PMID: 30833594
- PMCID: PMC6399277
- DOI: 10.1038/s41598-019-39677-2
In vivo investigation of hyperpolarized [1,3-13C2]acetoacetate as a metabolic probe in normal brain and in glioma
Abstract
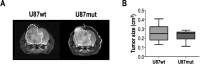
Dysregulation in NAD+/NADH levels is associated with increased cell division and elevated levels of reactive oxygen species in rapidly proliferating cancer cells. Conversion of the ketone body acetoacetate (AcAc) to β-hydroxybutyrate (β-HB) by the mitochondrial enzyme β-hydroxybutyrate dehydrogenase (BDH) depends upon NADH availability. The β-HB-to-AcAc ratio is therefore expected to reflect mitochondrial redox. Previous studies reported the potential of hyperpolarized 13C-AcAc to monitor mitochondrial redox in cells, perfused organs and in vivo. However, the ability of hyperpolarized 13C-AcAc to cross the blood brain barrier (BBB) and its potential to monitor brain metabolism remained unknown. Our goal was to assess the value of hyperpolarized [1,3-13C2]AcAc in healthy and tumor-bearing mice in vivo. Following hyperpolarized [1,3-13C2]AcAc injection, production of [1,3-13C2]β-HB was detected in normal and tumor-bearing mice. Significantly higher levels of [1-13C]AcAc and lower [1-13C]β-HB-to-[1-13C]AcAc ratios were observed in tumor-bearing mice. These results were consistent with decreased BDH activity in tumors and associated with increased total cellular NAD+/NADH. Our study confirmed that AcAc crosses the BBB and can be used for monitoring metabolism in the brain. It highlights the potential of AcAc for future clinical translation and its potential utility for monitoring metabolic changes associated with glioma, and other neurological disorders.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

