Deletion of Limk1 and Limk2 in mice does not alter cochlear development or auditory function
- PMID: 30833597
- PMCID: PMC6399249
- DOI: 10.1038/s41598-019-39769-z
Deletion of Limk1 and Limk2 in mice does not alter cochlear development or auditory function
Abstract
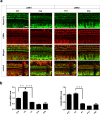
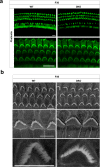
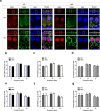
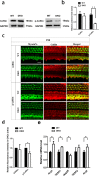
Inherited hearing loss is associated with gene mutations that result in sensory hair cell (HC) malfunction. HC structure is defined by the cytoskeleton, which is mainly composed of actin filaments and actin-binding partners. LIM motif-containing protein kinases (LIMKs) are the primary regulators of actin dynamics and consist of two members: LIMK1 and LIMK2. Actin arrangement is directly involved in the regulation of cytoskeletal structure and the maturation of synapses in the central nervous system, and LIMKs are involved in structural plasticity by controlling the activation of the actin depolymerization protein cofilin in the olfactory system and in the hippocampus. However, the expression pattern and the role of LIMKs in mouse cochlear development and synapse function also need to be further studied. We show here that the Limk genes are expressed in the mouse cochlea. We examined the morphology and the afferent synapse densities of HCs and measured the auditory function in Limk1 and Limk2 double knockout (DKO) mice. We found that the loss of Limk1 and Limk2 did not appear to affect the overall development of the cochlea, including the number of HCs and the structure of hair bundles. There were no significant differences in auditory thresholds between DKO mice and wild-type littermates. However, the expression of p-cofilin in the DKO mice was significantly decreased. Additionally, no significant differences were found in the number or distribution of ribbon synapses between the DKO and wild-type mice. In summary, our data suggest that the Limk genes play a different role in the development of the cochlea compared to their role in the central nervous system.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

