In Vivo Flow Cytometry of Extremely Rare Circulating Cells
- PMID: 30833671
- PMCID: PMC6399281
- DOI: 10.1038/s41598-019-40143-2
In Vivo Flow Cytometry of Extremely Rare Circulating Cells
Abstract
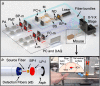
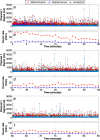
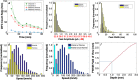
Circulating tumor cells (CTCs) are of great interest in cancer research, but methods for their enumeration remain far from optimal. We developed a new small animal research tool called "Diffuse in vivo Flow Cytometry" (DiFC) for detecting extremely rare fluorescently-labeled circulating cells directly in the bloodstream. The technique exploits near-infrared diffuse photons to detect and count cells flowing in large superficial arteries and veins without drawing blood samples. DiFC uses custom-designed, dual fiber optic probes that are placed in contact with the skin surface approximately above a major vascular bundle. In combination with a novel signal processing algorithm, DiFC allows counting of individual cells moving in arterial or venous directions, as well as measurement of their speed and depth. We show that DiFC allows sampling of the entire circulating blood volume of a mouse in under 10 minutes, while maintaining a false alarm rate of 0.014 per minute. In practice, this means that DiFC allows reliable detection of circulating cells below 1 cell per mL. Hence, the unique capabilities of DiFC are highly suited to biological applications involving very rare cell types such as the study of hematogenous cancer metastasis.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Bidard, F.-C. et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. The Lancet Oncology15, 406–414, 10.1016/S1470-2045(14)70069-5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

