Social evolution of innate immunity evasion in a virus
- PMID: 30833734
- PMCID: PMC6544518
- DOI: 10.1038/s41564-019-0379-8
Social evolution of innate immunity evasion in a virus
Abstract
Antiviral immunity has been studied extensively from the perspective of virus-cell interactions, yet the role of virus-virus interactions remains poorly addressed. Here, we demonstrate that viral escape from interferon (IFN)-based innate immunity is a social process in which IFN-stimulating viruses determine the fitness of neighbouring viruses. We propose a general and simple social evolution framework to analyse how natural selection acts on IFN shutdown and validate it in cell cultures and mice infected with vesicular stomatitis virus. Furthermore, we find that IFN shutdown is costly because it reduces short-term viral progeny production, thus fulfilling the definition of an altruistic trait. Hence, in well-mixed populations, the IFN-blocking wild-type virus is susceptible to invasion by IFN-stimulating variants and spatial structure consequently determines whether IFN shutdown can evolve. Our findings reveal that fundamental social evolution rules govern viral innate immunity evasion and virulence and suggest possible antiviral interventions.
Conflict of interest statement
Figures
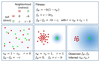

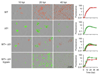


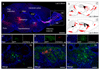
Comment in
-
Altruism in a virus.Nat Microbiol. 2019 Jun;4(6):910-911. doi: 10.1038/s41564-019-0463-0. Nat Microbiol. 2019. PMID: 31118503 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

