Dynamic changes of key metabolites during liver fibrosis in rats
- PMID: 30833800
- PMCID: PMC6397726
- DOI: 10.3748/wjg.v25.i8.941
Dynamic changes of key metabolites during liver fibrosis in rats
Abstract
Background: Fibrosis is the single most important predictor of significant morbidity and mortality in patients with chronic liver disease. Established non-invasive tests for monitoring fibrosis are lacking, and new biomarkers of liver fibrosis and function are needed.
Aim: To depict the process of liver fibrosis and look for novel biomarkers for diagnosis and monitoring fibrosis progression.
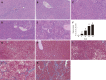
Methods: CCl4 was used to establish the rat liver fibrosis model. Liver fibrosis process was measured by liver chemical tests, liver histopathology, and Masson's trichrome staining. The expression levels of two fibrotic markers including α-smooth muscle actin and transforming growth factor β1 were assessed using immunohistochemistry and real-time polymerase chain reaction. Dynamic changes in metabolic profiles and biomarker concentrations in rat serum during liver fibrosis progression were investigated using ultra-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. The discriminatory capability of potential biomarkers was evaluated by receiver operating characteristic (ROC) curve analysis.
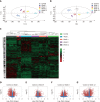
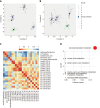
Results: To investigate the dynamic changes of metabolites during the process of liver fibrosis, sera from control and fibrosis model rats based on pathological results were analyzed at five different time points. We investigated the association of liver fibrosis with 21 metabolites including hydroxyethyl glycine, L-threonine, indoleacrylic acid, β-muricholic acid (β-MCA), cervonoyl ethanolamide (CEA), phosphatidylcholines, and lysophosphatidylcholines. Two metabolites, CEA and β-MCA, differed significantly in the fibrosis model rats compared to controls (P < 0.05) and showed prognostic value for fibrosis. ROC curve analyses performed to calculate the area under the curve (AUC) revealed that CEA and β-MCA differed significantly in the fibrosis group compared to controls with AUC values exceeding 0.8, and can clearly differentiate early stage from late stage fibrosis or cirrhosis.
Conclusion: This study identified two novel biomarkers of fibrosis, CEA and β-MCA, which were effective for diagnosing fibrosis in an animal model.
Keywords: Biomarker; Cervonoyl ethanolamide; Liver fibrosis; Metabonomics; Ultra-performance liquid chromatography-mass spectrometry; β-muricholic acid.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interest.
Figures
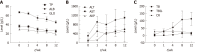


References
-
- Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: The simple becomes complex. Am J Physiol Gastrointest Liver Physiol. 2000;279:G7–G11. - PubMed
-
- Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology. 2002;36:S47–S56. - PubMed
-
- Chao DT, Lim JK, Ayoub WS, Nguyen LH, Nguyen MH. Systematic review with meta-analysis: The proportion of chronic hepatitis B patients with normal alanine transaminase ≤ 40 IU/L and significant hepatic fibrosis. Aliment Pharmacol Ther. 2014;39:349–358. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

