The Role of Iron in Friedreich's Ataxia: Insights From Studies in Human Tissues and Cellular and Animal Models
- PMID: 30833885
- PMCID: PMC6387962
- DOI: 10.3389/fnins.2019.00075
The Role of Iron in Friedreich's Ataxia: Insights From Studies in Human Tissues and Cellular and Animal Models
Abstract
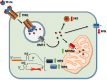
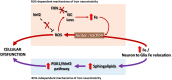
Friedreich's ataxia (FRDA) is a rare early-onset degenerative disease that affects both the central and peripheral nervous systems, and other extraneural tissues, mainly the heart and endocrine pancreas. This disorder progresses as a mixed sensory and cerebellar ataxia, primarily disturbing the proprioceptive pathways in the spinal cord, peripheral nerves and nuclei of the cerebellum. FRDA is an inherited disease with an autosomal recessive pattern caused by an insufficient amount of the nuclear-encoded mitochondrial protein frataxin, which is an essential and highly evolutionary conserved protein whose deficit results in iron metabolism dysregulation and mitochondrial dysfunction. The first experimental evidence connecting frataxin with iron homeostasis came from Saccharomyces cerevisiae; iron accumulates in the mitochondria of yeast with deletion of the frataxin ortholog gene. This finding was soon linked to previous observations of iron deposits in the hearts of FRDA patients and was later reported in animal models of the disease. Despite advances made in the understanding of FRDA pathophysiology, the role of iron in this disease has not yet been completely clarified. Some of the questions still unresolved include the molecular mechanisms responsible for the iron accumulation and iron-mediated toxicity. Here, we review the contribution of the cellular and animal models of FRDA and relevance of the studies using FRDA patient samples to gain knowledge about these issues. Mechanisms of mitochondrial iron overload are discussed considering the potential roles of frataxin in the major mitochondrial metabolic pathways that use iron. We also analyzed the effect of iron toxicity on neuronal degeneration in FRDA by reactive oxygen species (ROS)-dependent and ROS-independent mechanisms. Finally, therapeutic strategies based on the control of iron toxicity are considered.
Keywords: Friedreich’s ataxia; animal models; frataxin; iron; iron chelators; lipid deregulation; oxidative stress.
Figures
References
-
- Acquaviva F., De Biase I., Nezi L., Ruggiero G., Tatangelo F., Pisano C., et al. (2005). Extra-mitochondrial localisation of frataxin and its association with IscU1 during enterocyte-like differentiation of the human colon adenocarcinoma cell line Caco-2. J. Cell Sci. 118 3917–3924. 10.1242/jcs.02516 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

