Anti-vascular endothelial growth factor for neovascular age-related macular degeneration
- PMID: 30834517
- PMCID: PMC6419319
- DOI: 10.1002/14651858.CD005139.pub4
Anti-vascular endothelial growth factor for neovascular age-related macular degeneration
Abstract
Background: Age-related macular degeneration (AMD) is the most common cause of uncorrectable severe vision loss in people aged 55 years and older in the developed world. Choroidal neovascularization (CNV) secondary to AMD accounts for most cases of AMD-related severe vision loss. Intravitreous injection of anti-vascular endothelial growth factor (anti-VEGF) agents aims to block the growth of abnormal blood vessels in the eye to prevent vision loss and, in some instances, to improve vision.
Objectives: • To investigate ocular and systemic effects of, and quality of life associated with, intravitreous injection of three anti-VEGF agents (pegaptanib, ranibizumab, and bevacizumab) versus no anti-VEGF treatment for patients with neovascular AMD• To compare the relative effects of one of these anti-VEGF agents versus another when administered in comparable dosages and regimens SEARCH METHODS: To identify eligible studies for this review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), which contains the Cochrane Eyes and Vision Trials Register (searched January 31, 2018); MEDLINE Ovid (1946 to January 31, 2018); Embase Ovid (1947 to January 31, 2018); the Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to January 31, 2018); the International Standard Randomized Controlled Trials Number (ISRCTN) Registry (www.isrctn.com/editAdvancedSearch - searched January 31, 2018); ClinicalTrials.gov (www.clinicaltrials.gov - searched November 28, 2018); and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en - searched January 31, 2018). We did not impose any date or language restrictions in electronic searches for trials.
Selection criteria: We included randomized controlled trials (RCTs) that evaluated pegaptanib, ranibizumab, or bevacizumab versus each other or versus a control treatment (e.g. sham treatment, photodynamic therapy), in which participants were followed for at least one year.
Data collection and analysis: Two review authors independently screened records, extracted data, and assessed risks of bias. We contacted trial authors for additional data. We compared outcomes using risk ratios (RRs) or mean differences (MDs). We used the standard methodological procedures expected by Cochrane.
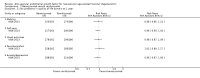
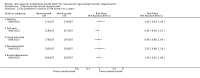
Main results: We included 16 RCTs that had enrolled a total of 6347 participants with neovascular AMD (the number of participants per trial ranged from 23 to 1208) and identified one potentially relevant ongoing trial. Six trials compared anti-VEGF treatment (pegaptanib, ranibizumab, or bevacizumab) versus control, and 10 trials compared bevacizumab versus ranibizumab. Pharmaceutical companies conducted or sponsored four trials but funded none of the studies that evaluated bevacizumab. Researchers conducted these trials at various centers across five continents (North and South America, Europe, Asia, and Australia). The overall certainty of the evidence was moderate to high, and most trials had an overall low risk of bias. All but one trial had been registered prospectively.When compared with those who received control treatment, more participants who received intravitreous injection of any of the three anti-VEGF agents had gained 15 letters or more of visual acuity (risk ratio [RR] 4.19, 95% confidence interval [CI] 2.32 to 7.55; moderate-certainty evidence), had lost fewer than 15 letters of visual acuity (RR 1.40, 95% CI 1.27 to 1.55; high-certainty evidence), and showed mean improvement in visual acuity (mean difference 6.7 letters, 95% CI 4.4 to 9.0 in one pegaptanib trial; mean difference 17.8 letters, 95% CI 16.0 to 19.7 in three ranibizumab trials; moderate-certainty evidence) after one year of follow-up. Participants treated with anti-VEGF agents showed improvement in morphologic outcomes (e.g. size of CNV, central retinal thickness) compared with participants not treated with anti-VEGF agents (moderate-certainty evidence). No trial directly compared pegaptanib versus another anti-VEGF agent and followed participants for one year; however, when compared with control treatments, ranibizumab and bevacizumab each yielded larger improvements in visual acuity outcomes than pegaptanib.Visual acuity outcomes after bevacizumab and ranibizumab were similar when the same RCTs compared the same regimens with respect to gain of 15 or more letters of visual acuity (RR 0.95, 95% CI 0.81 to 1.12; high-certainty evidence) and loss of fewer than 15 letters of visual acuity (RR 1.00, 95% CI 0.98 to 1.02; high-certainty evidence); results showed similar mean improvement in visual acuity (mean difference [MD] -0.5 letters, 95% CI -1.5 to 0.5; high-certainty evidence) after one year of follow-up, despite the substantially lower cost of bevacizumab compared with ranibizumab. Reduction in central retinal thickness was less among bevacizumab-treated participants than among ranibizumab-treated participants after one year (MD -11.6 μm, 95% CI -21.6 to -1.7; high-certainty evidence); however, this difference is within the range of measurement error, and we did not interpret it to be clinically meaningful.Ocular inflammation and increased intraocular pressure (IOP) after intravitreal injection were the most frequently reported serious ocular adverse events. Researchers reported endophthalmitis in less than 1% of anti-VEGF-treated participants and in no cases among control groups. The occurrence of serious systemic adverse events was comparable across anti-VEGF-treated groups and control groups; however, the numbers of events and trial participants may have been insufficient to show a meaningful difference between groups (evidence of low- to moderate-certainty). Investigators rarely measured and reported data on visual function, quality of life, or economic outcomes.
Authors' conclusions: Results of this review show the effectiveness of anti-VEGF agents (pegaptanib, ranibizumab, and bevacizumab) in terms of maintaining visual acuity; studies show that ranibizumab and bevacizumab improved visual acuity in some eyes that received these agents and were equally effective. Available information on the adverse effects of each medication does not suggest a higher incidence of potentially vision-threatening complications with intravitreous injection of anti-VEGF agents compared with control interventions; however, clinical trial sample sizes were not sufficient to estimate differences in rare safety outcomes. Future Cochrane Reviews should incorporate research evaluating variable dosing regimens of anti-VEGF agents, effects of long-term use, use of combination therapies (e.g. anti-VEGF treatment plus photodynamic therapy), and other methods of delivering these agents.
Conflict of interest statement
None known.
Figures
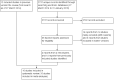


































Update of
-
Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2008 Apr 16;(2):CD005139. doi: 10.1002/14651858.CD005139.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2014 Aug 29;(8):CD005139. doi: 10.1002/14651858.CD005139.pub3. Update in: Cochrane Database Syst Rev. 2019 Mar 04;3:CD005139. doi: 10.1002/14651858.CD005139.pub4. PMID: 18425911 Free PMC article. Updated.
References
References to studies included in this review
ABC 2010 {published and unpublished data}
-
- Patel PJ, Chen FK, Cruz L, Rubin GS, Tufail A. Contrast sensitivity outcomes in the ABC Trial: a randomized trial of bevacizumab for neovascular age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2011;52(6):3089‐93. - PubMed
-
- Patel PJ, Henderson L, Sivaprasad S, Bunce C, Wormald R, Tufail A. The ABC trial ‐ a randomized double‐masked phase III study of the efficacy and safety of Avastin® (Bevacizumab) intravitreal injections compared to standard therapy in subjects with choroidal neovascularization secondary to age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐abstract 4536.
-
- Patel PJ, Rubin GS, Chen FK, Da‐Cruz L, Tufail A. Impact of anti‐vascular endothelial growth factor therapy on reading performance in age‐related macular degeneration: a randomized controlled trial. Investigative Ophthalmology and Visual Science 2015;56(7):4794.
-
- Patel PJ, Tufail A, Bunce C, Cruz L, Dowler J, Egan C, et al. A randomised, double‐masked phase III/IV study of the efficacy and safety of Avastin® (Bevacizumab) intravitreal injections compared to standard therapy in subjects with choroidal neovascularisation secondary to age‐related macular degeneration: clinical trial design. Trials 2008;9:56. - PMC - PubMed
ANCHOR 2006 {published and unpublished data}
-
- Alexander SG, Blodi BA, Webster MKW, Elledge JA, Hiner CJ, Armstrong J, et al. Ranibizumab (Lucentis®) in subjects with predominantly classic CNV: reading center evaluation of angiographic eligibility characteristics and baseline fluorescein angiographic data. Investigative Ophthalmology and Visual Science 2006;47:ARVO E‐abstract 2207.
-
- Barbazetto I, Saroj N, Shapiro H, Wong P, Freund KB. Dosing regimen and the frequency of macular hemorrhages in neovascular age‐related macular degeneration treated with ranibizumab. Retina 2010;30(9):1376‐85. - PubMed
-
- Barbazetto IA, Saroj N, Shapiro H, Wong P, Ho AC, Freund KB. Incidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trials. American Journal of Ophthalmology 2010;149(6):939‐46. - PubMed
-
- Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina 2012;32(9):1821‐8. - PubMed
-
- Bressler NM, Chang TS, Fine JT, Dolan CM, Ward J, Anti‐VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age‐Related Macular Degeneration (ANCHOR) Research Group. Improved vision‐related function after ranibizumab vs photodynamic therapy: a randomized clinical trial. Archives of Ophthalmology 2009;127(1):13‐21. - PubMed
Biswas 2011 {published data only}
BRAMD 2016 {published data only}
-
- Schauwvlieghe ASME, Dijkman G, Hooymans JMM, Verbraak FD, Dijkgraaf MG, Peto T, et al. Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2014;55(13):870.
CATT 2011 {published and unpublished data}
-
- Comparison of Age‐Related Macular Degeneration Treatment Trials. www.med.upenn.edu/cpob/studies/documents/CATTEligibilityCriteria.pdf (accessed 20 December 2011).
-
- CATT Research Group, Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE. Five‐year outcomes with anti‐vascular endothelial growth factor treatment of neovascular age‐related macular degeneration: the Comparison of Age‐Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123(8):1751‐61. - PMC - PubMed
GEFAL 2013 {published and unpublished data}
-
- Kodjikian L, Souied EH, Mimoun G, Mauget‐Faysse M, Behar‐Cohen F, Decullier E, et al. Ranibizumab versus bevacizumab for neovascular age‐related macular degeneration: results from the GEFAL noninferiority randomized trial. Ophthalmology 2013;120(11):2300‐9. - PubMed
IVAN 2013 {published and unpublished data}
-
- A randomised controlled trial of alternative treatments to inhibit VEGF in age‐related choroidal neovascularisation. www.ivan‐trial.co.uk/Default.aspx (accessed 4 April 2014).
-
- Erratum: Ranibizumab versus bevacizumab to treat neovascular age‐related macular degeneration: one‐year findings from the IVAN randomized trial (Ophthalmology (2012) 119 (1399‐1411)). Ophthalmology 2012;119(8):1508. - PubMed
-
- Chakravarthy U, Harding SP, Rogers CA, Downes S, Lotery AJ, Dakin HA, et al. A randomised controlled trial to assess the clinical effectiveness and cost‐effectiveness of alternative treatments to inhibit VEGF in age‐related choroidal neovascularisation (IVAN). Health Technology Assessment 2015;19(78):1‐298. - PMC - PubMed
-
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, on behalf of the IVAN study investigators. Alternative treatments to inhibit VEGF in age‐related choroidal neovascularisation: 2‐year findings of the IVAN randomised controlled trial. Lancet 2013;382(9900):1258‐67. - PubMed
-
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age‐related macular degeneration: one‐year findings from the IVAN randomized trial. Ophthalmology 2012;119(7):1399‐411. - PubMed
LUCAS 2015 {published and unpublished data}
-
- Berg K, Hadzalic E, Gjertsen I, Forsaa V, Berger LH, Kinge B, et al. Ranibizumab or bevacizumab for neovascular age‐related macular degeneration according to the Lucentis Compared to Avastin Study treat‐and‐extend protocol: two‐year results. Ophthalmology 2016;123(1):51‐9. - PubMed
-
- Berg K, Pedersen TR, Sandvik L, Bragadottir R. Comparison of ranibizumab and bevacizumab for neovascular age‐related macular degeneration according to LUCAS treat‐and extend protocol. Ophthalmology 2015;122(1):146‐52. - PubMed
MANTA 2013 {published and unpublished data}
-
- Krebs I, Schmetterer L, Boltz A, Told R, Vécsei‐Marlovits V, Egger S, et al. A randomised double‐masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age‐related macular degeneration. British Journal of Ophthalmology 2013;97(3):266‐71. - PubMed
MARINA 2006 {published and unpublished data}
-
- Barbazetto I, Saroj N, Shapiro H, Wong P, Freund KB. Dosing regimen and the frequency of macular hemorrhages in neovascular age‐related macular degeneration treated with ranibizumab. Retina 2010;30(9):1376‐85. - PubMed
-
- Barbazetto IA, Saroj N, Shapiro H, Wong P, Ho AC, Freund KB. Incidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trials. American Journal of Ophthalmology 2010;149(6):939‐46. - PubMed
-
- Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR, MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age‐related macular degeneration. Ophthalmology 2007;114(2):246‐52. - PubMed
-
- Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina 2012;32(9):1821‐8. - PubMed
-
- Bressler NM, Chang TS, Suñer IJ, Fine JT, Dolan CM, Ward J, et al. Vision‐related function after ranibizumab treatment by better‐ or worse‐seeing eye: clinical trial results from MARINA and ANCHOR. Ophthalmology 2010;117(4):747‐56. - PubMed
PIER 2008 {published and unpublished data}
-
- Abraham P, Yue H, Shams N. PIER: year 1 results of a phase IIIb study of ranibizumab efficacy and safety in choroidal neovascularization due to age‐related macular degeneration. European VitreoRetinal Society 2006. Symposium I: Anti‐Angiogenesis (Part 1). www.evrs.eu/2006‐evrs‐congress‐cannes (accessed 13 March 2013).
-
- Abraham P, Yue H, Wilson L. Randomized, double‐masked, sham‐controlled trial of ranibizumab for neovascular age‐related macular degeneration: PIER study year 2. American Journal of Ophthalmology 2010;150(3):315‐24. - PubMed
-
- Barbazetto I, Saroj N, Shapiro H, Wong P, Freund KB. Dosing regimen and the frequency of macular hemorrhages in neovascular age‐related macular degeneration treated with ranibizumab. Retina 2010;30(9):1376‐85. - PubMed
-
- Benz MS, Brown DM, Shapiro H, Tuomi L. Ranibizumab for neovascular age‐related macular degeneration (AMD): optical coherence tomography (OCT) vs. visual acuity (VA) changes in PIER study. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 347.
-
- Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, et al. Cerebrovascular accidents in patients treated for choroidal neovascularization with ranibizumab in randomized controlled trials. Retina 2012;32(9):1821‐8. - PubMed
Sacu 2009 {published and unpublished data}
-
- Michels SM, Weigert G, Geitzenauer W, Sacu S, Alina V, Schmidt‐Erfurth U. Intravitreal bevacizumab (Avastin®) therapy versus verteporfin therapy and intravitreal triamcinolone for neovascular age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2007;48:ARVO E‐Abstract 1820.
-
- Prager F, Michels S, Sacu S, Weigert G, Dunavölgyi R, Geitzenauer W, et al. Intravitreal bevacizumab (Avastin®) monotherapy versus photodynamic therapy plus intravitreal triamcinolone for neovascular age‐related macular degeneration: 12 months results of a prospective, randomized, controlled clinical trial. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 1168. - PubMed
-
- Sacu S, Michels S, Prager F, Weigert G, Dunavoelgyi R, Geitzenauer W, et al. Randomised clinical trial of intravitreal Avastin® vs photodynamic therapy and intravitreal triamcinolone: long‐term results. Eye 2009;23(12):2223‐7. - PubMed
-
- Weigert G, Michels S, Sacu S, Varga A, Prager F, Geitzenauer W, et al. Intravitreal bevacizumab (Avastin®) therapy versus photodynamic therapy plus intravitreal triamcinolone for neovascular age‐related macular degeneration: 6‐month results of a prospective, randomised, controlled clinical study. British Journal of Ophthalmology 2008;92(3):356‐60. - PubMed
SAVE‐AMD 2017 {published data only (unpublished sought but not used)}
Scholler 2014 {published data only}
-
- Scholler A, Richter‐Mueksch S, Weingessel B, Vecsei‐Marlovits P‐V. Differences of frequency in administration of ranibizumab and bevacizumab in patients with neovascular AMD. Wiener Klinische Wochenschrift (Central European Journal of Medicine) 2014;126:355‐9. - PubMed
Subramanian 2010 {published and unpublished data}
-
- Donahue SP, Recchia F, Sternberg P Jr. Bevacizumab vs ranibizumab for age‐related macular degeneration: early results of a prospective double‐masked, randomized clinical trial. American Journal of Ophthalmology 2010;150(2):287. - PubMed
-
- Messori A, Fadda V, Trippoli S. Randomized study of bevacizumab vs ranibizumab for age‐related macular degeneration: inappropriate conclusions. American Journal of Ophthalmology 2010;149(5):867. - PubMed
-
- Subramanian ML, Abedi G, Ness S, Ahmed E, Fenberg M, Daly MK, et al. Bevacizumab vs ranibizumab for age‐related macular degeneration: 1‐year outcomes of a prospective, double‐masked randomised clinical trial. Eye 2010;24(11):1708‐15. - PubMed
-
- Subramanian ML, Ness S, Abedi G, Ahmed E, Daly M, Feinberg E, et al. Bevacizumab vs ranibizumab for age‐related macular degeneration: early results of a prospective double‐masked, randomized clinical trial. American Journal of Ophthalmology 2009;148(6):875‐82. - PubMed
-
- Subramanian ML, Ness S, Abedi G, Ahmed E, Daly M, Feinberg E, et al. Reply. American Journal of Ophthalmology 2010;149(5):867.
VISION 2004 {published and unpublished data}
-
- Chakravarthy U. Efficacy and safety of pegaptanib in age‐related macular degeneration: three‐year results of the V.I.S.I.O.N. trials. The Macula Society. 2006:116.
-
- D'Amico DF. Macugen® in neovascular age‐related macular degeneration: exploratory subgroup analyses. American Academy of Ophthalmology. 2005:261.
-
- D'Amico DJ. VEGF Inhibition Study in Ocular Neovascularization (VISION): second year efficacy data. Investigative Ophthalmology and Visual Science 2005;46:ARVO E‐abstract 2309.
-
- D'Amico DJ, Bird AC. VEGF Inhibition Study in Ocular Neovascularization‐1 (VISION‐1): safety evaluation from the pivotal Macugen® (pegaptanib sodium) clinical trials. Investigative Ophthalmology and Visual Science 2004;45:ARVO E‐Abstract 2363.
-
- Eyetech Pharmaceuticals, Inc, Pfizer. Inc. Pegaptanib sodium injection in the treatment of neovascular age‐related macular degeneration. www.fda.gov/ohrms/dockets/ac/04/briefing/2004‐4053b1.htm (accessed 20 December 2011).
References to studies excluded from this review
ADVANCE 2008 {unpublished data only}
-
- Safety and efficacy of oral PTK787 in patients with subfoveal choroidal neovascularization secondary to age‐related macular degeneration (AMD) (ADVANCE). ClinicalTrials.gov NCT00138632. [NCT00531336]
ARMAST 2008 {unpublished data only}
-
- Photodynamic therapy combined with bevacizumab vs bevacizumab alone for neovascular age‐related macular degeneration (ARMAST). ClinicalTrials.gov NCT0069592.
Bashshur 2007 {published data only}
-
- Bashshur ZF, Schakal A, Hamam RN, Haibi CP, Jaafar R, Noureddin BN. Intravitreal bevacizumab vs verteporfin photodynamic therapy for neovascular age‐related macular degeneration. Archives of Ophthalmology 2007;125(10):1357‐61. - PubMed
BEAT‐AMD 2009 {published data only}
-
- Schmid‐Kubista KE, Krebs I, Gruenberger B, Schueller J, Binder S. Systemic bevacizumab therapy for exudative neovascular age‐related macular degeneration (BEAT‐AMD‐Study). Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 304. - PubMed
-
- Schmid‐Kubista KE, Krebs I, Gruenberger B, Zeiler F, Schueller J, Binder S. Systemic bevacizumab (Avastin®) therapy for exudative neovascular age‐related macular degeneration. The BEAT‐AMD‐Study. British Journal of Ophthalmology 2009;93(7):914‐9. - PubMed
Berger 2015 {published data only}
-
- Berger BB, Yanni SE, Wenzel A, Weichselberger A, Hubschman JP. Efficacy of RTH258 (ESBA1008), an anti‐VEGF agent, applied by microvolume injection or infusion in subjects with neovascular AMD. Investigative Ophthalmology and Visual Science 2015;56 (7):821.
Blaha 2015 {published data only}
-
- Blaha GR, Brooks NO, Mackel CE, Pani A, Stewart AP, Price LL, et al. Changes in flare after intravitreal injection of three different anti‐vascular endothelial growth factor medications. Retina 2015;35(3):577‐81. - PubMed
Bolz 2008 {published data only}
-
- Bolz M, Pruente C, Benesch T, Ritter M, Deak G, Golbaz I, et al. The relevance of measuring central retinal thickness during intra‐vitreal therapy with ranibizumab: analyzing a multi‐center clinical trial. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 5576.
Brown 2016 {published data only}
-
- Brown DM, Ingerman A, Shearn SP, Slakter JS. CNV lesion characteristics as a predictor of visual outcome in wet AMD patients receiving combination therapy of intravitreal anti‐VEGF therapy and topical Squalamine lactate ophthalmic solution. Investigative Ophthalmology and Visual Science 2016;57(12):4419.
Cohen 2008 {published data only}
-
- Cohen SY, Bremond‐Gignac D, Quentel G, Mimoun G, Citterio T, Bisot‐Locard S, et al. Cost‐effectiveness sequential modeling of ranibizumab versus usual care in age‐related macular degeneration. Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(11):1527‐34. - PubMed
Costagliola 2010 {published data only}
-
- Costagliola C, Romano MR, Rinaldi M, dell'Omo R, Chiosi F, Menzione M, Semeraro F. Low fluence rate photodynamic therapy combined with intravitreal bevacizumab for neovascular age‐related macular degeneration. British Journal of Ophthalmology 2010;94(2):180‐4. - PubMed
Csaky 2015 {published data only}
-
- Csaky KG, Dugel PU, Pierce AJ, Fries MA, Kelly DS, Danis RP, et al. Clinical evaluation of pazopanib eye drops versus ranibizumab intravitreal injections in subjects with neovascular age‐related macular degeneration. Ophthalmology 2015;122(3):579‐88. - PubMed
Earnshaw 2007 {published data only}
-
- Earnshaw SR, Moride Y, Rochon S. Cost‐effectiveness of pegaptanib compared to photodynamic therapy with verteporfin and to standard care in the treatment of subfoveal wet age‐related macular degeneration in Canada. Clinical Therapeutics 2007;29(9):2096‐106. - PubMed
Eibenberger 2015 {published data only}
-
- Eibenberger K, Sulzbacher F, Rezar S, Roberts PK, Sacu S, Schmidt‐Erfurth U. Randomized comparison of anterior chamber inflammatory activity in eyes treated with intravitreal aflibercept or ranibizumab. Investigative Ophthalmology and Visual Science 2015;56(7):1517.
EMERGE 2016 {published data only}
-
- Christmas NJ. A phase 2 study (EMERGE) evaluating repeated intravitreal administration of ICON‐1 in patients with choroidal neovascularization (CNV) secondary to age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2016;57(12):4434.
-
- NCT02358889. Study evaluating intravitreal hl‐con1TM in patients with ehoroidal neovascularization secondary to age‐related macular degeneration (EMERGE). ClinicalTrials.gov (accessed 24 August 2018).
-
- Wells JA, Gonzale CR, Berger BB, Gonzalez VH, Sippy BD, Burian G. A phase I, open‐label, dose‐excalation trial to investigate safety and tolerability of single intravitreous injections of ICON‐1 targeting tissue factor in wet AMD. Ophthalmic Surgery, Lasers and Imaging Retina 2018;49:336‐45. [DOI 10.3928/23258160‐20180501‐07; PUBMED: PMID 29772044] - PubMed
Erdokur 2009 {published data only}
-
- Erdokur O, Tetikoglu M, Ozturk M, Elcioglu M. Results of comparison in use to alternative therapy methods for subfoveal choroidal neovascularization secondary to age‐related macular degeneration. Retina‐Vitreus 2009;17(4):245‐50.
EXTEND‐I 2008 {published data only}
-
- Tano Y. The safety and efficacy of ranibizumab in Japanese patients with subfoveal choroidal neovascularization secondary to age‐related macular degeneration: 12‐month results from the phase I/II EXTEND‐I study. Investigative Ophthalmology and Visual Science 2008;49:ARVO E‐Abstract 272.
-
- Tano Y, Ohji M, EXTEND‐I Study Group. EXTEND‐I: safety and efficacy of ranibizumab in Japanese patients with subfoveal choroidal neovascularization secondary to age‐related macular degeneration. Acta Ophthalmologica 2010;88(3):309‐16. - PubMed
Eyetech Study 2003 {published data only}
-
- Eyetech Study Group. Anti‐vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age‐related macular degeneration: phase II study results. Ophthalmology 2003;110(5):979‐86. - PubMed
Falkenstein 2007 {published data only}
Fletcher 2008 {published data only}
-
- Fletcher EC, Lade RJ, Adewoyin T, Chong NV. Computerized model of cost‐utility analysis for treatment of age‐related macular degeneration. Ophthalmology 2008;115(12):2192‐8. - PubMed
FOCUS 2006 {published and unpublished data}
-
- Antoszyk AN, Tuomi L, Chung CY, Singh A, FOCUS Study Group. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age‐related macular degeneration (FOCUS): year 2 results. American Journal of Ophthalmology 2008;145(5):862‐74. - PubMed
-
- Heier JS, Boyer DS, Ciulla TA, Ferrone PJ, Jumber MJ, Gentile RC, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age‐related macular degeneration: year 1 results of the FOCUS study. Archives of Ophthalmology 2006;124(11):1532‐42. - PubMed
-
- Heier JS, FOCUS Study Group. Intravitreal ranibizumab with verteporfin photodynamic therapy for neovascular age‐related macular degeneration: year one results. Program and abstracts of the American Society of Retina Specialists 23rd Annual Meeting; 2005 July 16‐20; Montreal. 2005.
GALATIR 2014 {unpublished data only}
-
- NCT02036723. Multicentre double blind randomized clinical study evaluating the efficacy and safety of BCD‐021 (CJSC BIOCAD, Russia) and Lucentis® (Novartis Pharmaceuticals Canada Inc.) in patients with neovascular wet age‐related macular degeneration (GALATIR). clinicaltrials.gov/show/NCT02036723 (accessed 5 February 2017).
Gallemore 2016 {published data only}
-
- Gallemore RP, Elman MJ, Milton M, Zamiri P, Grosskreutz CL. A proof of concept study of intravitreal (IVT) LFG316 in patients with neovascular age related macular degeneration (NAMD). Investigative Ophthalmology and Visual Science 2016;57(12):4985.
Hahn 2007 {published data only}
-
- Hahn R, Sacu S, Michels S, Varga A, Weigert G, Geitzenauer W, et al. Intravitreal bevacizumab versus verteporfin and intravitreal triamcinolone acetonide in patients with neovascular age‐related macula degeneration. Der Ophthalmologe 2007;104(7):588‐93. - PubMed
Hatta 2010 {published data only}
-
- Hatta Y, Ishikawa K, Nishihara H, Ozawa S, Ito Y, Terasaki H. Effect of photodynamic therapy alone or combined with posterior subtenon triamcinolone acetonide or intravitreal bevacizumab on choroidal hypofluorescence by indocyanine green angiography. Retina 2010;30(3):495‐502. - PubMed
HAWK 2018 {published data only}
-
- Dugel PU, Warburton J, Weichselberger A, Sallstig P. A 2‐year study comparing the efficacy and safety of brolucizumab vs aflibercept in subjects with neovascular age‐related macular degeneration: testing an alternative treatment regimen. Investigative Ophthalmology and Visual Science 2016;57(12):5018.
Heier 2006 {published data only}
-
- Heier JS, Antoszyk AN, Pavan PR, Leff SR, Rosenfeld PJ, Ciulla TA, et al. Ranibizumab for treatment of neovascular age‐related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology 2006;113(4):642. e1‐4. - PubMed
Hernandez‐Pastor 2008 {published data only}
-
- Hernandez‐Pastor LJ, Ortega A, Garcia‐Layana A, Giraldez J. Cost‐effectiveness of ranibizumab compared with photodynamic treatment of neovascular age‐related macular degeneration. Clinical Therapeutics 2008;30(12):2436‐51. - PubMed
Hernandez‐Pastor 2010 {published data only}
-
- Hernandez‐Pastor LJ, Ortega A, Garcia‐Layana A, Giraldez J. Cost‐effectiveness of ranibizumab compared with pegaptanib in neovascular age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2010;248(4):467‐76. - PubMed
Holz 2016 {published data only}
-
- Holz FG, Dugel PU, Weissgerber G, Hamilton R, Silva R, Bandello F, et al. Single‐chain antibody fragment VEGF inhibitor RTH258 for neovascular age‐related macular degeneration: a randomized controlled study. Ophthalmology 2016;123(5):1080‐9. - PubMed
Javitt 2008 {published data only}
-
- Javitt JC, Zlateva GP, Earnshaw SR, Pleil AM, Graham CN, Brogan AJ, et al. Cost‐effectiveness model for neovascular age‐related macular degeneration: comparing early and late treatment with pegaptanib sodium based on visual acuity. Value in Health 2008;11(4):563‐74. - PubMed
Kralinger 2014 {published data only}
-
- Kralinger MT, Zehetner C, Waltl I, Kirchmayr R, Kieselbach GF. VEGF Plasma levels after intravitreal injection of aflibercept and ranibizumab. Investigative Ophthalmology and Visual Science 2014;55(13):4965.
Lai 2009 {published data only}
-
- Lai TY, Liu DT, Chan KP, Luk FO, Pang CP, Lam DS. Visual outcomes and growth factor changes of two dosages of intravitreal bevacizumab for neovascular age‐related macular degeneration: a randomized, controlled trial. Retina 2009;29(9):1218‐26. - PubMed
Lazic 2007 {published data only}
-
- Lazic R, Gabric N. Verteporfin therapy and intravitreal bevacizumab combined and alone in choroidal neovascularization due to age‐related macular degeneration. Ophthalmology 2007;114(6):1179‐85. - PubMed
Li 2012 {published data only}
-
- Li X, Hu Y, Sun X, Zhang J, Zhang M, Neovascular Age‐Related Macular Degeneration Treatment Trial Using Bevacizumab (NATTB). Bevacizumab for neovascular age‐related macular degeneration in China. Ophthalmology 2012;119(10):2087‐93. - PubMed
Li 2013 {published data only}
Matthe 2011 {published data only}
-
- Matthe E, Sandner D. Monotherapy of exudative age‐related macular degeneration with ranibizumab in patients at cardiovascular risk. Advantages of ranibizumab compared to a combination with pegaptanib. Der Ophthalmologe 2011;108(4):337‐41. - PubMed
MIRA‐1 2005 {published data only}
-
- Pulido JS, Anderson WB Jr, Flynn JT, Lichter PR, Sanders D. Multicenter prospective, randomized, double‐masked, placebo‐controlled study of rheopheresis to treat neovascular age‐related macular degeneration: interim analysis. Transactions of the American Ophthalmological Society 2002;100:85‐107. - PMC - PubMed
-
- Pulido JS, Sanders D, Klingel R. Rheopheresis for age‐related macular degeneration: clinical results and putative mechanism of action. Canadian Journal of Ophthalmology 2005;40(3):332‐40. - PubMed
Modarres 2009 {published data only}
-
- Modarres M, Naseripour M, Falavarjani KG, Nikeghbali A, Hashemi M, Parvaresh MM. Intravitreal injection of 2.5 mg versus 1.25 mg bevacizumab (Avastin®) for treatment of CNV associated with AMD. Retina 2009;29(3):319‐24. - PubMed
NCT00087763 {unpublished data only}
-
- NCT00087763. A phase II prospective, randomized, double‐masked, sham‐controlled, dose‐ranging, multi‐center trial to assess the effect of pegaptanib sodium on foveal thickening in patients with exudative subfoveal age‐related macular degeneration (AMD). clinicaltrials.gov/show/NCT00087763 (accessed 5 February 2017).
NCT01494805 {published data only}
-
- Lai CM, Magno A, Pierce C, Samulski RJ, Chalberg TW, Blumenkranz MS, et al. Results from a phase I/II clinical trial on anti‐vascular endothelial growth factor gene therapy in patients with exudative age‐related macular degeneration. Journal of Gene Medicine 2013;15(8‐9):319.
-
- Lai CM, Magno AL, Barone SB, Schwartz SD, Blumenkranz MS, Constable IJ, et al. Optical coherence tomography profiles, visual acuity and ranibizumab usage during 3‐year follow‐up in a rAAV.sFLT‐1 gene therapy clinical trial for wet age‐related macular degeneration. Molecular therapy. Conference: 20th Annual Meeting of the American Society of Gene and Cell Therapy, ASGCT 2017. United States 2017;25:315.
-
- Rakoczy EP, Lai CM, Magno A, French M, Barone SB, Schwartz SD, et al. Association of serum neutralizing antibodies with safety and efficacy outcomes in a Phase 2a trial of rAAV.sFlt‐ 1 in the treatment of wet AMD. Investigative Ophthalmology and Visual Science 2016;57(12):526.
NCT01796964 {published data only}
-
- Dugel PU, Jaffe GJ, Salistig P, Warburton J, Weichselberger A, Wieland M, et al. Brolucizumab versus aflibercept in participants with neovascular age‐related macular degeneration: a randomized trial. Ophthalmology 2017;124(9):1296‐304. - PubMed
-
- Dugel PU, Jaffe GJ, Sallstig P, Warburton J, Weichselberger A, Wieland M, et al. Brolucizumab versus aflibercept in participants with neovascular age‐related macular degeneration: a randomized trial. Ophthalmology 2017;124(9):1296‐304. - PubMed
Neubauer 2007 {published data only}
-
- Neubauer AS, Holz FG, Schrader W, Back EI, Kuhn T, Hirneiss C, et al. Cost‐utility analysis of ranibizumab (Lucentis®) in neovascular macular degeneration. Klinische Monatsblatter fur Augenheilkunde 2007; Vol. 224, issue 9:727‐32. - PubMed
Nguyen 2006 {published data only}
-
- Nguyen QD, Shah SM, Hafiz G, Quinlan E, Sung J, Chu K, et al. A phase I trial of an IV‐administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age‐related macular degeneration. Ophthalmology 2006;113(9):1522. - PubMed
Nowak 2012 {published data only}
-
- Nowak MS, Jurowski P, Grzybowski A, Goś R, Pastuszka M, Kapica A, et al. A prospective study on different methods for the treatment of choroidal neovascularization. The efficacy of verteporfin photodynamic therapy, intravitreal bevacizumab and transpupillary thermotherapy in patients with neovascular age‐related macular degeneration. Medical Science Monitor 2012;18(6):CR374‐80. - PMC - PubMed
Nunes 2014 {published data only}
-
- Nunes RP, Rodrigues E, Hirai FE, Barroso LF, Novais E, Badaro E, et al. Efficacy and cost‐effectiveness of anti‐VEGF treatments for age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2014;55(13):4939.
OSPREY 2014 {published data only}
-
- Singerman LJ, Weichselberger A, Sallstig P. OSPREY trial: randomized, active‐controlled, phase II study to evaluate safety and efficacy of RTH258, a humanized single chain anti‐VEGF antibody fragment, in patients with neovascular AMD. Investigative Ophthalmology and Visual Science 2015;56(7):4801.
Parodi 2012 {published data only}
-
- Parodi MB, Cascavilla M, Papayannis A, Kontadakis DS, Bandello F, Iacono P. Intravitreal bevacizumab in advanced‐stage neovascular age‐related macular degeneration with visual acuity lower than 20/200. Archives of Ophthalmology 2012;130(7):934‐5. - PubMed
PERSPECTIVES 2012 {published data only}
-
- Chakravarthy U, Staurenghi G, Kwok K, Tressler CS, Buggage R, PERSPECTIVES Study Group. Treating early choroidal neovascularisation with pegaptanib sodium in patients with neovascular age‐related macular degeneration: findings of the PERSPECTIVES study. British Journal of Ophthalmology 2012;96(10):1351‐4. - PubMed
PrONTO Study 2009 {published data only}
-
- Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable‐dosing regimen with intravitreal ranibizumab for neovascular age‐related macular degeneration: year 2 of the PrONTO study. American Journal of Ophthalmology 2009;148(1):43‐58.e1. [10.1016/j.ajo.2009.01.024.Epub 2009 Apr 18] - PubMed
Raftery 2007 {published data only}
RATE 2011 {unpublished data only}
-
- NCT01319188. Ranibizumab for age‐related macular degeneration and the risk of arterial thromboembolic events (RATE). clinicaltrials.gov/show/NCT01319188 (accessed 5 February 2017).
RIVAL 2017 {published data only}
-
- Gillies MC, Hunyor AP, Arnold JJ, Pecheur F, Underhill K, Guymer RH, et al. Comparison of ranibizumab and aflibercept in patients with neovascular age‐related macular degeneration treated following a 'treat and extend' protocol: efficacy variables from the pre‐specified 12‐month interim analysis of the rival study. Clinical and Experimental Ophthalmology. Conference: 49th Annual Scientific Congress of the Royal Australian and New Zealand College of Ophthalmologists. Australia 2017;45(1):29.
SAILOR 2009 {published data only}
-
- Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age‐related macular degeneration. Ophthalmology 2009;116(9):1731‐9. - PubMed
-
- Reichel E, Francom S, Rubio R. Ranibizumab (Lucentis®) safety in previously treated and newly diagnosed patients with neovascular age‐related macular degeneration (AMD): the SAILOR study. The Macula Society. 2008:168.
Schmid‐Kubista 2011 {published and unpublished data}
-
- Schmid‐Kubista KE, Krebs I, Ansari‐Shahrezaei S, Haas P, Hagen S, Binder S. Comparing treatment of neovascular age‐related macular degeneration with sequential intravitreal Avastin and Macugen versus intravitreal mono‐therapy ‐ a pilot study. Current Eye Research 2011;36(10):958‐63. - PubMed
Sengul 2017 {published data only}
-
- Sengul A, Rasier R, Ciftci C, Artunay O, Kockar A, Bahcecioglu H, et al. Short‐term effects of intravitreal ranibizumab and bevacizumab administration on 24‐h ambulatory blood pressure monitoring recordings in normotensive patients with age‐related macular degeneration. Eye (London, England) 2017;31(5):677‐83. - PMC - PubMed
SIGHT 2014 {published data only}
-
- Li X, Chen Y, Zhang J, Xu X, Zhang F, Cheung CMG, et al. Intravitreal aflibercept versus photodynamic therapy in Chinese patients with neovascular age‐related macular degeneration: outcomes of the sight study. Journal of Ocular Pharmacology and Therapeutics 2017;33(6):435‐44.
SUMMIT 2007 {published data only}
-
- Slakter JS, DENALI Study Group. Combination therapy with verteporfin PDT and ranibizumab for subfoveal choroidal neovascularization due to AMD. Investigative Ophthalmology and Visual Science 2007;48:E‐Abstract 1817.
Suñer 2009 {published data only}
-
- Suñer IJ, Kokame GT, Yu E, Ward J, Dolan C, Bressler NM. Responsiveness of NEI VFQ‐25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Investigative Ophthalmology and Visual Science 2009;50(8):3629‐35. - PubMed
Tano 2008 {published data only}
-
- Tano Y, Pegaptanib Sodium Multi‐center Study Group. Pegaptanib sodium one‐year treatment study for neovascular age‐related macular degeneration. Nippon Ganka Gakkai Zasshi 2008;112(7):590‐600. - PubMed
Vallance 2010 {published data only}
-
- Vallance JH, Johnson B, Majid MA, Banerjee S, Mandal K, Bailey CC. A randomised prospective double‐masked exploratory study comparing combination photodynamic treatment and intravitreal ranibizumab vs intravitreal ranibizumab monotherapy in the treatment of neovascular age‐related macular degeneration. Eye 2010;24(10):1561‐7. - PubMed
VERITAS 2006 {published data only}
-
- Mieler WF, The VERITAS trial. VERITAS ‐ The rationale and design of a combination therapy trial for wet AMD. Investigative Ophthalmology and Visual Science 2006;47:E‐Abstract 5232.
VIEW 2014 {published data only}
-
- Marcus DM. Long‐term follow‐up of intravitreal aflibercept injection (IAI) for neovascular age‐related macular degeneration (nAMD) in an open‐label extension of the VIEW1 study. Investigative Ophthalmology and Visual Science. 2014; Vol. 55(13):3943.
-
- Schmidt‐Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age‐related macular degeneration: ninety‐six‐week results of the VIEW studies. Ophthalmology 2014;121(1):193‐201. - PubMed
Weigert 2008 {published data only}
-
- Weigert G, Michels S, Sacu S, Varga A, Prager F, Geitzenauer W, et al. Intravitreal bevacizumab (Avastin) versus photodynamic therapy plus intravitreal triamcinolone for neovascular age‐related macular degeneration: 6‐month results of a prospective, randomized controlled clinical trial. British Journal of Ophthalmology 2008;92(3):356‐8. [PUBMED: PMID: 18201136] - PubMed
Wolowacz 2007 {published data only}
-
- Wolowacz SE, Roskell N, Kelly S, Maciver FM, Brand CS. Cost effectiveness of pegaptanib for the treatment of age‐related macular degeneration in the UK. Pharmacoeconomics 2007;25(10):863‐79. - PubMed
Zehetner 2013 {published data only}
-
- Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age‐related macular degeneration, and in patients with diabetic macular oedema. British Journal of Ophthalmology 2013;97(4):454‐9. - PubMed
References to ongoing studies
NCT00559715 {unpublished data only}
-
- NCT00559715. Prevention of vision loss in patients with age‐related macular degeneration (AMD) by intravitreal injection of bevacizumab and ranibizumab (VIBERA). clinicaltrials.gov/show/NCT00559715 (accessed 22 August 2018).
Additional references
Aiello 1994
-
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. New England Journal of Medicine 1994;331(22):1480‐7. - PubMed
AREDS 2001
-
- Age‐Related Eye Disease Study Research Group. A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E, beta carotene, and zinc for age‐related macular degeneration and vision loss: AREDS report no. 8. Archives of Ophthalmology 2001;119(10):1414‐36. - PMC - PubMed
AREDS2 2013
-
- Age‐Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega‐3 fatty acids for age‐related macular degeneration: the Age‐Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309(19):2005‐15. - PubMed
Boekhoorn 2007
-
- Boekhoorn SS, Vingerling JR, Witteman JCM, Hofman A, Jong PT. C‐reactive protein level and risk of aging macula disorder: the Rotterdam study. Archives of Ophthalmology 2007;125(10):1396‐401. - PubMed
Bourne 2014
-
- Bourne RR, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, et al. Prevalence and causes of vision loss in high‐income countries and in Eastern and Central Europe: 1990‐2010. British Journal of Ophthalmology 2014;98(5):629‐38. - PubMed
Brown 2008
-
- Brown MM, Brown GC, Brown HC, Peet J. A value‐based medicine analysis of ranibizumab for the treatment of subfoveal neovascular macular degeneration. Ophthalmology 2008;115(6):1039‐45. - PubMed
Bunce 2006
Carneiro 2012
CMS 2014
-
- Centers for Medicare and Medicaid Services. Billing and coding information regarding uses, including off‐label uses, of bevacizumab and ranibizumab, for the treatment of ophthalmological diseases. Document ID A49034. www.cms.gov/medicare‐coverage‐database (accessed 9 April 2014).
Cohen 2014
-
- Cohen D. Roche and Novartis colluded over wet AMD drugs, says Italian regulator. BMJ 2014;348:g2006. - PubMed
Congdon 2004
-
- Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, et al. Causes and prevalence of visual impairment among adults in the United States. Archives of Ophthalmology 2004;122(4):477‐85. - PubMed
de Juan 2013
-
- Juan E, Laganovska G, Loewenstein A, Alster Y, Erickson S. First in‐human results of a refillable drug delivery implant providing sustained release of ranibizumab in patients with neovascular AMD. Macula Society. 2013:136‐7.
Deangelis 2007
-
- Deangelis MM, Ji F, Kim IK, Adams S, Capone A Jr, Ott J, et al. Cigarette smoking, CFH, APOE, ELOVL4, and risk of neovascular age‐related macular degeneration. Archives of Ophthalmology 2007;125(1):49‐54. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9. Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
EOP 1003
-
- Eyetech Pharmaceuticals, Inc, Pfizer, Inc. Pegaptanib sodium injection in the treatment of neovascular age‐related macular degeneration; and FDA. Macugen® (pegaptanib sodium injection) for the treatment of neovascular age‐related macular degeneration. www.fda.gov/ohrms/dockets/ac/04/briefing/2004‐4053b1.htm (accessed 20 December 2011).
EOP 1004
-
- Eyetech Pharmaceuticals, Inc, Pfizer, Inc. Pegaptanib sodium injection in the treatment of neovascular age‐related macular degeneration; and FDA. Macugen® (pegaptanib sodium injection) for the treatment of neovascular age‐related macular degeneration. www.fda.gov/ohrms/dockets/ac/04/briefing/2004‐4053b1.htm (accessed 20 December 2011).
Friedman 2004
-
- Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, Jong PT, et al. Prevalence of age‐related macular degeneration in the United States. Archives of Ophthalmology 2004;122(4):564‐72. - PubMed
Ghafour 1983
Gillies 2014
-
- Gillies MC, Walton RJ, Arnold JJ, McAllister IL, Simpson JM, Hunyor AP, et al. Comparison of outcomes from a phase 3 study of age‐related macular degeneration with a matched, observational cohort. Ophthalmology 2014;121(3):676‐81. - PubMed
Glanville 2006
GRADEpro 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro. Version accessed 11 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Gragoudas 2004
-
- Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR, the VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age‐related macular degeneration. New England Journal of Medicine 2004;351(27):2805‐16. - PubMed
Haddad 2006
-
- Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age‐related macular degeneration: a review of progress to date. Survey of Ophthalmology 2006;51(4):316‐63. - PubMed
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holz 2013
-
- Holz FG, Bandello F, Gillies M, Mitchell P, Osborne A, Sheidow T, et al. Safety of ranibizumab in routine clinical practice: 1‐year retrospective pooled analysis of four European neovascular AMD registries within the LUMINOUS programme. British Journal of Ophthalmology 2013;97(9):1161‐7. - PMC - PubMed
Hyman 1987
-
- Hyman L. Epidemiology of eye disease in the elderly. Eye 1987;1(Pt 2):330‐41. - PubMed
Ip 2008
-
- Ip MS, Scott IU, Brown GC, Brown MM, Ho AC, Huang SS, et al. Anti‐vascular endothelial growth factor pharmacotherapy for age‐related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology 2008;115(10):1837‐46. - PubMed
Klein 2008
-
- Klein R, Knudtson MD, Cruickshanks KJ, Klein BE. Further observations on the association between smoking and the long‐term incidence and progression of age‐related macular degeneration. Archives of Ophthalmology 2008;126(1):115‐21. - PubMed
Kvanta 1996
-
- Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age‐related macular degeneration express vascular endothelial growth factor. Investigative Ophthalmology and Visual Science 1996;37(9):1929‐34. - PubMed
Leibowitz 1980
-
- Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973‐1975. Survey of Ophthalmology 1980;24(Suppl):335‐610. - PubMed
Li 2016
Lopez 1996
-
- Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age‐related macular degeneration‐related choroidal neovascular membranes. Investigative Ophthalmology and Visual Science 1996;37(5):855‐68. - PubMed
Mitchell 2002
-
- Mitchell P, Wang JJ, Smith W, Leeder SR. Smoking and the 5‐year incidence of age‐related maculopathy: the Blue Mountains Eye Study. Archives of Ophthalmology 2002;120(10):1357‐63. - PubMed
Mitchell 2011
-
- Mitchell P. A systematic review of the efficacy and safety outcomes of anti‐VEGF agents used for treating neovascular age‐related macular degeneration: comparison of ranibizumab and bevacizumab. Current Medical Research and Opinion 2011;27(7):1465‐75. - PubMed
Moja 2014
MPSG 1991
-
- Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy: five‐year results from randomized clinical trials. Archives of Ophthalmology 1991;109(8):1109‐14. - PubMed
Owen 2003
Rasmussen 2014
-
- Rasmussen A, Sander B. Long‐term longitudinal study of patients treated with ranibizumab for neovascular age‐related macular degeneration. Current Opinion in Ophthalmology 2014;25(3):158‐63. - PubMed
Review Manager 5 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rofagha 2013
-
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven‐year outcomes in ranibizumab‐treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN‐UP). Ophthalmology 2013;120(11):2292‐9. - PubMed
Sarwar 2016
Schaumberg 2007
-
- Schaumberg DA, Hankinson SE, Guo Q, Rimm E, Hunter DJ. A prospective study of 2 major age‐related macular degeneration susceptibility alleles and interactions with modifiable risk factors. Archives of Ophthalmology 2007;125(1):55‐62. - PubMed
Schmucker 2010
-
- Schmucker C, Ehlken C, Hansen LL, Antes G, Agostini HT, Lelgemann M. Intravitreal bevacizumab (Avastin) vs. ranibizumab (Lucentis) for the treatment of age‐related macular degeneration: a systematic review. Current Opinion in Ophthalmology 2010;21(3):218‐26. - PubMed
Schmucker 2012
Seddon 2006
-
- Seddon JM, George S, Rosner B, Klein ML. CFH gene variant, Y402H, and smoking, body mass index, environmental associations with advanced age‐related macular degeneration. Human Heredity 2006;61(3):157‐65. - PubMed
Smith 1996
-
- Smith W, Mitchell P, Leeder SR. Smoking and age‐related maculopathy. The Blue Mountains Eye Study. Archives of Ophthalmology 1996;114(12):1518‐23. - PubMed
SST 20
-
- Submacular Surgery Trials Research Group, Solomon SD, Jefferys JL, Hawkins BS, Bressler NM. Incident choroidal neovascularization in fellow eyes of patients with unilateral subfoveal choroidal neovascularization secondary to age‐related macular degeneration: SST report No. 20 from the Submacular Surgery Trials Research Group. Archives of Ophthalmology 2007;125(10):1323‐30. - PubMed
Stein 2014
Tielsch 1994
-
- Tielsch JA. Vision problems in the US: a report on blindness and vision impairment in adults age 40 and older. Prevent Blindness. Illinois: Schaumberg, 1994.
Ting 2002
-
- Ting TD, Oh M, Cox TA, Meyer CH, Toth CA. Decreased visual acuity associated with cystoid macular edema in neovascular age‐related macular degeneration. Archives of Ophthalmology 2002;120(6):731‐7. - PubMed
Van Kerckhoven 2001
-
- Kerckhoven W, Lafaut B, Follens I, Laey JJ. Features of age‐related macular degeneration on optical coherence tomography. Bulletin de la Societe Belge d'Ophtalmologie 2001;281:75‐84. - PubMed
Virgili 2007
References to other published versions of this review
Krzystolik 2005
Solomon 2014
Solomon 2016
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

