Effect of sonic hedgehog on motor neuron positioning in the spinal cord during chicken embryonic development
- PMID: 30834718
- PMCID: PMC6484327
- DOI: 10.1111/jcmm.14254
Effect of sonic hedgehog on motor neuron positioning in the spinal cord during chicken embryonic development
Abstract
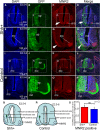
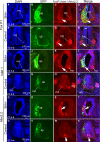
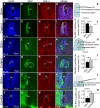
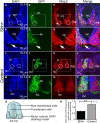
Sonic hedgehog (SHH) is a vertebrate homologue of the secreted Drosophila protein hedgehog and is expressed by the notochord and floor plate in the developing spinal cord. Sonic hedgehog provides signals relevant for positional information, cell proliferation and possibly cell survival, depending on the time and location of expression. Although the role of SHH in providing positional information in the neural tube has been experimentally proven, the underlying mechanism remains unclear. In this study, in ovo electroporation was employed in the chicken spinal cord during chicken embryo development. Electroporation was conducted at stage 17 (E2.5), after electroporation the embryos were continued incubating to stage 28 (E6) for sampling, tissue fixation with 4% paraformaldehyde and frozen sectioning. Sonic hedgehog and related protein expressions were detected by in situ hybridization and fluorescence immunohistochemistry and the results were analysed after microphotography. Our results indicate that the ectopic expression of SHH leads to ventralization in the spinal cord during chicken embryonic development by inducing abnormalities in the structure of the motor column and motor neuron integration. In addition, ectopic SHH expression inhibits the expression of dorsal transcription factors and commissural axon projections. The correct location of SHH expression is vital to the formation of the motor column. Ectopic expression of SHH in the spinal cord not only affects the positioning of motor neurons, but also induces abnormalities in the structure of the motor column. It leads to ventralization in the spinal cord, resulting in the formation of more ventral neurons forming during neuronal formation.
Keywords: chicken embryo; in ovo electroporation; motor neuron; sonic hedgehog; spinal cord.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare that they have no known conflicts of interest associated with this publication.
Figures



References
-
- Cayuso J, Ulloa F, Cox B, Briscoe J, Martí E. The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development. 2006;133:517‐528. - PubMed
-
- Pringle NP, Yu WP, Guthrie S, et al. Determination of neuroepithelial cell fate: induction of the oligodendrocyte lineage by ventral midline cells and sonic hedgehog. Dev Biol. 1996;177:30‐42. - PubMed
-
- Yamada T, Placzek M, Tanaka H, Dodd J, Jessell TM. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991;64:635‐647. - PubMed
-
- Yamada T, Pfaff SL, Edlund T, Jessell TM. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1993;73:673‐686. - PubMed
-
- Tanabe Y, Roelink H, Jessell TM. Induction of motor neurons by sonic hedgehog is independent of floor plate differentiation. Curr Biol. 1995;5:651‐658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

