Comparison of phosphorescent agents for noninvasive sensing of tumor oxygenation via Cherenkov-excited luminescence imaging
- PMID: 30834723
- PMCID: PMC6397946
- DOI: 10.1117/1.JBO.24.3.036001
Comparison of phosphorescent agents for noninvasive sensing of tumor oxygenation via Cherenkov-excited luminescence imaging
Abstract
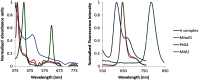
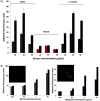
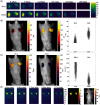
Cherenkov emission generated in tissue during radiotherapy can be harnessed for the imaging biochemistry of tissue microenvironments. Cherenkov-excited luminescence scanned imaging (CELSI) provides a way to optically and noninvasively map oxygen-related signals, which is known to correlate to outcomes in radiotherapy. Four candidate phosphorescent reagents PtG4, MM2, Ir(btb)2 ( acac ) , and MitoID were studied for oxygen sensing, testing in a progressive series of (a) in solution, (b) in vitro, and (c) in subcutaneous tumors. In each test, the signal strength and response to oxygen were assessed by phosphorescence intensity and decay lifetime measurement. MM2 showed the most robust response to oxygen changes in solution, followed by PtG4, Ir(btb)2 ( acac ) , and MitoID. However, in PANC-1 cells, their oxygen responses differed with Ir(btb)2 ( acac ) exhibiting the largest phosphorescent intensity change in response to changes in oxygenation, followed by PtG4, MM2, and MitoID. In vivo, it was only possible to utilize Ir(btb)2 ( acac ) and PtG4, with each being used at nanomole levels, to determine signal strength, lifetime, and pO2. Oxygen sensing with CELSI during radiotherapy is feasible and can estimate values from 1 mm regions of tissue when used in the configuration of this study. PtG4 was the most amenable to in vivo sensing on the timescale of external beam LINAC x-rays.
Keywords: Cherenkov radiation; optical imaging; phosphorescence; tumor hypoxia.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

