Evidence for a role for Sestrin1 in mediating leucine-induced activation of mTORC1 in skeletal muscle
- PMID: 30835510
- PMCID: PMC6580170
- DOI: 10.1152/ajpendo.00522.2018
Evidence for a role for Sestrin1 in mediating leucine-induced activation of mTORC1 in skeletal muscle
Abstract
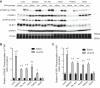
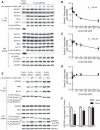
Previous studies established that leucine stimulates protein synthesis in skeletal muscle to the same extent as a complete mixture of amino acids, and the effect occurs through activation of the mechanistic target of rapamycin in complex 1 (mTORC1). Recent studies using cells in culture showed that the Sestrins bind leucine and are required for leucine-dependent activation of mTORC1. However, the role they play in mediating leucine-dependent activation of the kinase in vivo has been questioned because the dissociation constant of Sestrin2 for leucine is well below circulating and intramuscular levels of the amino acid. The goal of the present study was to compare expression of the Sestrins in skeletal muscle to other tissues and to assess their relative role in mediating activation of mTORC1 by leucine. The results show that the relative expression of the Sestrin proteins varies widely among tissues and that in skeletal muscle Sestrin1 expression is higher than Sestrin3, whereas Sestrin2 expression is markedly lower. Analysis of the dissociation constants of the Sestrins for leucine as assessed by leucine-induced dissociation of the Sestrin·GAP activity toward Rags 2 (GATOR2) complex revealed that Sestrin1 has the highest affinity for leucine and that Sestrin3 has the lowest affinity. In agreement with the dissociation constants calculated using cells in culture, oral leucine administration promotes disassembly of the Sestrin1·GATOR2 complex but not the Sestrin2 or Sestrin3·GATOR2 complex. Overall, the results presented herein are consistent with a model in which leucine-induced activation of mTORC1 in skeletal muscle in vivo occurs primarily through release of Sestrin1 from GATOR2.
Keywords: Sestrin; leucine; mTOR; skeletal muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340: 1100–1106, 2013. doi: 10.1126/science.1232044. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

