Noninvasive detection of graft injury after heart transplant using donor-derived cell-free DNA: A prospective multicenter study
- PMID: 30835940
- PMCID: PMC6790566
- DOI: 10.1111/ajt.15339
Noninvasive detection of graft injury after heart transplant using donor-derived cell-free DNA: A prospective multicenter study
Abstract
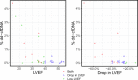
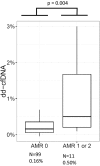
Standardized donor-derived cell-free DNA (dd-cfDNA) testing has been introduced into clinical use to monitor kidney transplant recipients for rejection. This report describes the performance of this dd-cfDNA assay to detect allograft rejection in samples from heart transplant (HT) recipients undergoing surveillance monitoring across the United States. Venous blood was longitudinally sampled from 740 HT recipients from 26 centers and in a single-center cohort of 33 patients at high risk for antibody-mediated rejection (AMR). Plasma dd-cfDNA was quantified by using targeted amplification and sequencing of a single nucleotide polymorphism panel. The dd-cfDNA levels were correlated to paired events of biopsy-based diagnosis of rejection. The median dd-cfDNA was 0.07% in reference HT recipients (2164 samples) and 0.17% in samples classified as acute rejection (35 samples; P = .005). At a 0.2% threshold, dd-cfDNA had a 44% sensitivity to detect rejection and a 97% negative predictive value. In the cohort at risk for AMR (11 samples), dd-cfDNA levels were elevated 3-fold in AMR compared with patients without AMR (99 samples, P = .004). The standardized dd-cfDNA test identified acute rejection in samples from a broad population of HT recipients. The reported test performance characteristics will guide the next stage of clinical utility studies of the dd-cfDNA assay.
Keywords: biomarker; clinical research/practice; heart (allograft) function/dysfunction; heart transplantation/cardiology.
© 2019 The Authors. American Journal of Transplantation published by Wiley Periodicals, Inc. on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures


References
-
- Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty‐fourth Adult Heart Transplantation Report‐2017; Focus Theme: allograft ischemic time. J Heart Lung Transplant. 2017;36(10):1037‐1046. - PubMed
-
- Baraldi‐Junkins C, Levin HR, Kasper EK, Rayburn BK, Herskowitz A, Baughman KL. Complications of endomyocardial biopsy in heart transplant patients. J Heart Lung Transplant. 1993;12(1 Pt 1):63‐67. - PubMed
-
- Bhat G, Burwig S, Walsh R. Morbidity of endomyocardial biopsy in cardiac transplant recipients. Am Heart J. 1993;125(4):1180‐1181. - PubMed
-
- Williams MJ, Lee MY, DiSalvo TG, et al. Biopsy‐induced flail tricuspid leaflet and tricuspid regurgitation following orthotopic cardiac transplantation. Am J Cardiol. 1996;77(15):1339‐1344. - PubMed
-
- Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene‐expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362(20):1890‐1900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

