Glucocorticoid Dosages and Acute-Phase Reactant Levels at Giant Cell Arteritis Flare in a Randomized Trial of Tocilizumab
- PMID: 30835950
- PMCID: PMC6772126
- DOI: 10.1002/art.40876
Glucocorticoid Dosages and Acute-Phase Reactant Levels at Giant Cell Arteritis Flare in a Randomized Trial of Tocilizumab
Abstract
Objective: This study was undertaken to evaluate glucocorticoid dosages and serologic findings in patients with giant cell arteritis (GCA) flares.
Methods: Patients with GCA were randomly assigned to receive double-blind dosing with either subcutaneous tocilizumab (TCZ) 162 mg weekly plus 26-week prednisone taper (TCZ-QW + Pred-26), every-other-week TCZ plus 26-week prednisone taper (TCZ-Q2W + Pred-26), placebo plus 26-week prednisone taper (PBO + Pred-26), or placebo plus 52-week prednisone taper (PBO + Pred-52). Outcome measures were prednisone dosage, C-reactive protein (CRP) level, and erythrocyte sedimentation rate (ESR) at the time of flare.
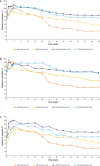
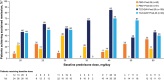
Results: One hundred patients received TCZ-QW + Pred-26, 49 received TCZ-Q2W + Pred-26, 50 received PBO + Pred-26, and 51 received PBO + Pred-52. Of the 149 TCZ-treated patients, 36 (24%) experienced flare, 23 (64%) of whom were still receiving prednisone (median dosage 2.0 mg/day). Among 101 PBO + Pred-treated patients, 59 (58%) experienced flare, 45 (76%) of whom were receiving prednisone (median dosage 5.0 mg/day). Many flares occurred while patients were taking >10 mg/day prednisone: 9 (25%) in the TCZ groups and 13 (22%) in the placebo groups. Thirty-three flares (92%) in TCZ-treated groups and 20 (34%) in PBO + Pred-treated groups occurred with normal CRP levels. More than half of the PBO + Pred-treated patients had elevated CRP levels without flares. Benefits of the TCZ and prednisone combination over prednisone alone for remission induction were apparent by 8 weeks.
Conclusion: Most GCA flares occurred while patients were still receiving prednisone. Acute-phase reactant levels were not reliable indicators of flare in patients treated with TCZ plus prednisone or with prednisone alone. The addition of TCZ to prednisone facilitates earlier GCA control.
Trial registration: ClinicalTrials.gov NCT01791153.
© 2019 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures

References
-
- Salvarani C, Pipitone N, Versari A, Hunder GG. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol 2012;8:509–21. - PubMed
-
- Gonzalez‐Gay MA, Martinez‐Dubois C, Agudo M, Pompei O, Blanco R, Llorca J. Giant cell arteritis: epidemiology, diagnosis, and management. Curr Rheumatol Rep 2010;12:436–42. - PubMed
-
- Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, et al. A multicenter, randomized, double‐blind, placebo‐controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002;46:1309–18. - PubMed
-
- Hoffman GS, Cid MC, Rendt‐Zagar KE, Merkel PA, Weyand CM, Stone JH, et al. Infliximab for maintenance of glucocorticosteroid‐induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007;146:621–30. - PubMed
-
- Seror R, Baron G, Hachulla E, Debandt M, Larroche C, Puéchal X, et al. Adalimumab for steroid sparing in patients with giant‐cell arteritis: results of a multicentre randomised controlled trial. Ann Rheum Dis 2014;73:2074–81. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

