Glucosylation and Glutathione Conjugation of Chlorpyrifos and Fluopyram Metabolites Using Electrochemistry/Mass Spectrometry
- PMID: 30836697
- PMCID: PMC6429400
- DOI: 10.3390/molecules24050898
Glucosylation and Glutathione Conjugation of Chlorpyrifos and Fluopyram Metabolites Using Electrochemistry/Mass Spectrometry
Abstract
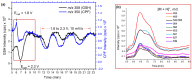
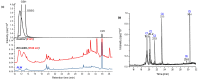
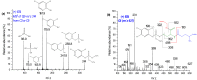
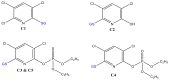
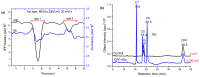
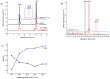
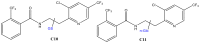
Xenobiotics and their reactive metabolites are conjugated with native biomolecules such as glutathione and glucoside during phase II metabolism. Toxic metabolites are usually detoxified during this step. On the other hand, these reactive species have a potential health impact by disrupting many enzymatic functions. Thus, it is crucial to understand phase II conjugation reactions of xenobiotics in order to address their fate and possible toxicity mechanisms. Additionally, conventional methods (in vivo and in vitro) have limitation due to matrix complexity and time-consuming. Hence, developing fast and matrix-free alternative method is highly demandable. In this work, oxidative phase I metabolites and reactive species of chlorpyrifos (insecticide) and fluopyram (fungicide) were electrochemically produced by using a boron-doped diamond electrode coupled online to electrospray mass spectrometry (ESI-MS). Reactive species of the substrates were trapped by biomolecules (glutathione and glucoside) and phase II conjugative metabolites were identified using liquid chromatography (LC)-MS/MS, and/or Triple time of flight (TripleTOF)-MS. Glutathione conjugates and glucosylation of chlorpyrifos, trichloropyridinol, oxon, and monohydroxyl fluopyram were identified successfully. Glutathione and glucoside were conjugated with chlorpyrifos, trichloropyridinol, and oxon by losing a neutral HCl. In the case of fluopyram, its monohydroxyl metabolite was actively conjugated with both glutathione and glucoside. In summary, seven bioconjugates of CPF and its metabolites and two bioconjugates of fluopyram metabolites were identified using electrochemistry (EC)/MS for the first time in this work. The work could be used as an alternative approach to identify glutathione and glucosylation conjugation reactions of other organic compounds too. It is important, especially to predict phase II conjugation within a short time and matrix-free environment.
Keywords: EC/MS; bioconjugation; glucosylation; glutathione; oxidative metabolism; pesticide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
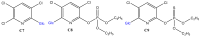
References
-
- Macherey A.-C., Dansette P.M. The Practice of Medicinal Chemistry. 5th ed. Academic Press; San Diego, CA, USA: 2015. Chapter 25—Biotransformations leading to toxic metabolites: Chemical aspects; pp. 585–614.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

