MBP-FGF2-Immobilized Matrix Maintains Self-Renewal and Myogenic Differentiation Potential of Skeletal Muscle Stem Cells
- PMID: 30836735
- PMCID: PMC6657940
- DOI: 10.15283/ijsc18125
MBP-FGF2-Immobilized Matrix Maintains Self-Renewal and Myogenic Differentiation Potential of Skeletal Muscle Stem Cells
Abstract
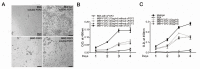
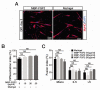
The robust capacity of skeletal muscle stem cells (SkMSCs, or satellite cells) to regenerate into new muscles in vivo has offered promising therapeutic options for the treatment of degenerative muscle diseases. However, the practical use of SkMSCs to treat muscle diseases is limited, owing to their inability to expand in vitro under defined cultivation conditions without loss of engraftment efficiency. To develop an optimal cultivation condition for SkMSCs, we investigated the behavior of SkMSCs on synthetic maltose-binding protein (MBP)-fibroblast growth factor 2 (FGF2)-immobilized matrix in vitro. We found that the chemically well-defined, xeno-free MBP-FGF2-immobilized matrix effectively supports SkMSC growth without reducing their differentiation potential in vitro. Our data highlights the possible application of the MBP-FGF2 matrix for SkMSC expansion in vitro.
Keywords: MBP-FGF2; Myogenic differentiation; Satellite cell; Self-renewal; Skeletal muscle stem cell.
Conflict of interest statement
The authors have no competing financial interest and potential conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

