Overall survival, costs, and healthcare resource use by line of therapy in Medicare patients with newly diagnosed metastatic urothelial carcinoma
- PMID: 30836812
- PMCID: PMC7384456
- DOI: 10.1080/13696998.2019.1591424
Overall survival, costs, and healthcare resource use by line of therapy in Medicare patients with newly diagnosed metastatic urothelial carcinoma
Abstract
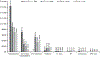
Aims: Medicare patients with metastatic or surgically unresectable urothelial carcinoma (mUC) often receive platinum-based chemotherapy as first line of therapy (LOT), but invariably progress, requiring additional LOTs and healthcare resource use (HCRU). To better understand the evolving mUC treatment landscape, the economic burden of chemotherapy-based mUC treatments among US Medicare patients was estimated. Methods: Newly diagnosed Medicare patients with mUC were identified from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database. Patients were followed from diagnosis to death, disenrollment, or end of study to characterize LOTs (first [LOT1], second [LOT2], and third or greater [LOT3+]). Kaplan-Meier methods were used to estimate overall survival (OS) by LOT. HCRU and mean costs were reported over the follow-up period, LOT duration, and maximum LOT received. Results: Among 1,873 eligible patients with mUC (median age = 77 years; median follow-up = 7.5 months), 1,035 (55%) received no chemotherapy. Among chemotherapy-treated patients, 61% had LOT1 only, 25% had LOT1 and LOT2 only, and 14% had LOT3+. Median OS was 8.1 months, range was 4.3 (untreated) to 29.8 (LOT3+) months. HCRU frequency increased with additional LOTs. Mean cumulative per-patient cost was $82,912 for all patients, increasing with additional LOTs (untreated = $57,207; LOT1 = $99,213; LOT2 = $125,190; LOT3+ = $163,884). Mean per patient per month cost was $18,827 for all patients, decreasing with increasing number of LOTs received (untreated = $27,211; LOT1 = $9,601; LOT2 = $7,325; LOT3+ = $6,017). Limitations: Potential for treatment misclassification when using the algorithm defining LOTs and non-generalizability of results to younger patients. Conclusions: Over 50% of Medicare patients with mUC received no chemotherapy. Among chemotherapy-treated patients, most received only one LOT. Additional LOTs led to higher mean costs and HCRU, but as patients were followed longer, monthly costs decreased. As treatments evolve to include immuno-oncology agents, these findings provide a clinically relevant economic benchmark for mUC treatment across different traditional LOTs.
Keywords: A10; E37; Metastatic urothelial carcinoma; SEER-Medicare; economic burden; healthcare resource use; line of therapy.
Figures


Similar articles
-
Real-world survival and economic burden among patients with locally advanced or metastatic urothelial carcinoma in the United States.Urol Oncol. 2025 Mar;43(3):189.e9-189.e18. doi: 10.1016/j.urolonc.2024.11.010. Epub 2024 Dec 14. Urol Oncol. 2025. PMID: 39675951
-
Real-world treatment patterns and outcomes of patients with unresectable or metastatic urothelial carcinoma receiving systemic therapy in Japan.Urol Oncol. 2025 May;43(5):329.e1-329.e8. doi: 10.1016/j.urolonc.2024.09.020. Epub 2024 Oct 15. Urol Oncol. 2025. PMID: 39414520
-
Factors associated with receipt of systemic anticancer treatment for locally advanced or metastatic urothelial carcinoma in England: a population-based study.Urol Oncol. 2024 Dec;42(12):451.e11-451.e18. doi: 10.1016/j.urolonc.2024.07.010. Epub 2024 Jul 28. Urol Oncol. 2024. PMID: 39069443
-
Platinum-based adjuvant chemotherapy for upper tract urothelial carcinoma: a change of paradigm? A meta-analysis of aggregate data.Anticancer Drugs. 2022 Jan 1;33(1):e61-e68. doi: 10.1097/CAD.0000000000001200. Anticancer Drugs. 2022. PMID: 34387596
-
Management of Metastatic Urothelial Carcinoma in Emerging Markets (EM): An Expert Opinion.Clin Genitourin Cancer. 2024 Apr;22(2):467-475. doi: 10.1016/j.clgc.2024.01.001. Epub 2024 Jan 3. Clin Genitourin Cancer. 2024. PMID: 38228413 Review.
Cited by
-
Clinical evidence and insights supporting the use of avelumab first-line maintenance treatment in patients with advanced urothelial carcinoma in the Asia-Pacific region.Asia Pac J Clin Oncol. 2022 Oct;18(5):e191-e203. doi: 10.1111/ajco.13765. Epub 2022 Mar 3. Asia Pac J Clin Oncol. 2022. PMID: 35238147 Free PMC article. Review.
-
Cost-Effectiveness and Economic Impact of Bladder Cancer Management: An Updated Review of the Literature.Pharmacoeconomics. 2023 Jul;41(7):751-769. doi: 10.1007/s40273-023-01273-8. Epub 2023 Apr 23. Pharmacoeconomics. 2023. PMID: 37088844 Review.
-
Treatment Patterns and Attrition With Lines of Therapy for Advanced Urothelial Carcinoma in the US.JAMA Netw Open. 2024 May 1;7(5):e249417. doi: 10.1001/jamanetworkopen.2024.9417. JAMA Netw Open. 2024. PMID: 38696168 Free PMC article.
-
The Impact of Progression on Healthcare Resource Utilization and Costs Among Patients with High-Grade Non-Muscle Invasive Bladder Cancer After Bacillus Calmette-Guérin Therapy: A Retrospective SEER-Medicare Analysis.Adv Ther. 2021 Mar;38(3):1584-1600. doi: 10.1007/s12325-020-01616-3. Epub 2021 Jan 11. Adv Ther. 2021. PMID: 33543424
-
Atezolizumab plus platinum-based chemotherapy as first-line therapy for metastatic urothelial cancer: A cost-effectiveness analysis.Front Pharmacol. 2022 Aug 22;13:872196. doi: 10.3389/fphar.2022.872196. eCollection 2022. Front Pharmacol. 2022. PMID: 36071854 Free PMC article.
References
-
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71:96–108. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Spiess PE, Agarwal N, Bangs R, et al. Bladder cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1240–1267. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
