The small and large ribosomal subunits depend on each other for stability and accumulation
- PMID: 30837296
- PMCID: PMC6402506
- DOI: 10.26508/lsa.201800150
The small and large ribosomal subunits depend on each other for stability and accumulation
Erratum in
-
Correction: The small and large ribosomal subunits depend on each other for stability and accumulation.Life Sci Alliance. 2019 Aug 1;2(4):e201900508. doi: 10.26508/lsa.201900508. Print 2019 Aug. Life Sci Alliance. 2019. PMID: 31371523 Free PMC article. No abstract available.
Abstract
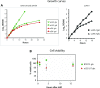
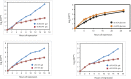
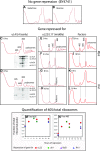
The 1:1 balance between the numbers of large and small ribosomal subunits can be disturbed by mutations that inhibit the assembly of only one of the subunits. Here, we have investigated if the cell can counteract an imbalance of the number of the two subunits. We show that abrogating 60S assembly blocks 40S subunit accumulation. In contrast, cessation of the 40S pathways does not prevent 60S accumulation, but does, however, lead to fragmentation of the 25S rRNA in 60S subunits and formation of a 55S ribosomal particle derived from the 60S. We also present evidence suggesting that these events occur post assembly and discuss the possibility that the turnover of subunits is due to vulnerability of free subunits not paired with the other subunit to form 80S ribosomes.
© 2019 Gregory et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







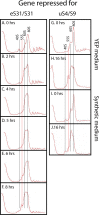
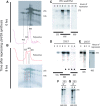

Similar articles
-
An open interface in the pre-80S ribosome coordinated by ribosome assembly factors Tsr1 and Dim1 enables temporal regulation of Fap7.RNA. 2021 Feb;27(2):221-233. doi: 10.1261/rna.077610.120. Epub 2020 Nov 20. RNA. 2021. PMID: 33219089 Free PMC article.
-
Co-Assembly of 40S and 60S Ribosomal Proteins in Early Steps of Eukaryotic Ribosome Assembly.Int J Mol Sci. 2019 Jun 8;20(11):2806. doi: 10.3390/ijms20112806. Int J Mol Sci. 2019. PMID: 31181743 Free PMC article.
-
RNA mimicry by the fap7 adenylate kinase in ribosome biogenesis.PLoS Biol. 2014 May 13;12(5):e1001860. doi: 10.1371/journal.pbio.1001860. eCollection 2014 May. PLoS Biol. 2014. PMID: 24823650 Free PMC article.
-
Inside the 40S ribosome assembly machinery.Curr Opin Chem Biol. 2011 Oct;15(5):657-63. doi: 10.1016/j.cbpa.2011.07.023. Epub 2011 Aug 20. Curr Opin Chem Biol. 2011. PMID: 21862385 Free PMC article. Review.
-
Nuclear export and cytoplasmic maturation of ribosomal subunits.FEBS Lett. 2007 Jun 19;581(15):2783-93. doi: 10.1016/j.febslet.2007.05.013. Epub 2007 May 11. FEBS Lett. 2007. PMID: 17509569 Review.
Cited by
-
The induction of p53 correlates with defects in the production, but not the levels, of the small ribosomal subunit and stalled large ribosomal subunit biogenesis.Nucleic Acids Res. 2023 Sep 22;51(17):9397-9414. doi: 10.1093/nar/gkad637. Nucleic Acids Res. 2023. PMID: 37526268 Free PMC article.
-
Spatial Transcriptomics and Single-Nucleus Multi-Omics Analysis Revealing the Impact of High Maternal Folic Acid Supplementation on Offspring Brain Development.Nutrients. 2024 Nov 7;16(22):3820. doi: 10.3390/nu16223820. Nutrients. 2024. PMID: 39599606 Free PMC article.
-
Progressive alterations in polysomal architecture and activation of ribosome stalling relief factors in a mouse model of Huntington's disease.Neurobiol Dis. 2024 Jun 1;195:106488. doi: 10.1016/j.nbd.2024.106488. Epub 2024 Mar 31. Neurobiol Dis. 2024. PMID: 38565397 Free PMC article.
-
Molecular Interplay between Non-Host Resistance, Pathogens and Basal Immunity as a Background for Fatal Yellowing in Oil Palm (Elaeis guineensis Jacq.) Plants.Int J Mol Sci. 2023 Aug 18;24(16):12918. doi: 10.3390/ijms241612918. Int J Mol Sci. 2023. PMID: 37629099 Free PMC article.
-
Functional Interactions of Ribosomal Intersubunit Bridges in Saccharomyces cerevisiae.Genetics. 2019 Dec;213(4):1329-1339. doi: 10.1534/genetics.119.302777. Epub 2019 Oct 24. Genetics. 2019. PMID: 31649153 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
