Distinct representations of basic taste qualities in human gustatory cortex
- PMID: 30837463
- PMCID: PMC6401093
- DOI: 10.1038/s41467-019-08857-z
Distinct representations of basic taste qualities in human gustatory cortex
Abstract
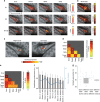
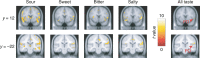
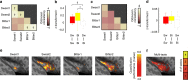
The mammalian tongue contains gustatory receptors tuned to basic taste types, providing an evolutionarily old hedonic compass for what and what not to ingest. Although representation of these distinct taste types is a defining feature of primary gustatory cortex in other animals, their identification has remained elusive in humans, leaving the demarcation of human gustatory cortex unclear. Here we used distributed multivoxel activity patterns to identify regions with patterns of activity differentially sensitive to sweet, salty, bitter, and sour taste qualities. These were found in the insula and overlying operculum, with regions in the anterior and middle insula discriminating all tastes and representing their combinatorial coding. These findings replicated at super-high 7 T field strength using different compounds of sweet and bitter taste types, suggesting taste sensation specificity rather than chemical or receptor specificity. Our results provide evidence of the human gustatory cortex in the insula.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

