Degradation of p47 by autophagy contributes to CADM1 overexpression in ATLL cells through the activation of NF-κB
- PMID: 30837480
- PMCID: PMC6400899
- DOI: 10.1038/s41598-019-39424-7
Degradation of p47 by autophagy contributes to CADM1 overexpression in ATLL cells through the activation of NF-κB
Abstract
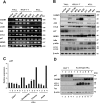
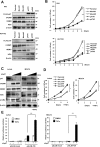
Cell adhesion molecule 1 (CADM1), a member of the immunoglobulin superfamily, is identified as a novel cell surface marker for human T-cell leukemia virus (HTLV-1)-infected T cells. Adult T-cell leukemia/lymphoma (ATLL) is developed in HTLV-1-infected T-cells after a long infection period. To examine the mechanism of CADM1 overexpression in ATLL, we first identified that CADM1 is transcriptionally up-regulated by a transcriptional enhancer element through NF-κB signaling pathway. In HTLV-1-infected T-cells, CADM1 expression is dependent on HTLV-1/Tax through activation of canonical and non-canonical NF-κB; however, in ATLL cells with frequent loss of Tax expression, the activation of canonical NF-κB only enhances the CADM1 expression. Along with active mutations in signaling molecules under T-cell recepor (TCR) signaling, degradation of p47, a negative regulator of NF-κB, was essential for activation of canonical NF-κB through stabilization of NEMO (NF-κB essential modulator). The mechanism of p47 degradation is primarily dependent on activation of lysosomal-autophagy and the autophagy is activated in most of the HTLV-infected and ATLL cells, suggesting that the p47 degradation may be a first key molecular event during HTLV-1 infection to T-cells as a connector of two important signaling pathways, NF-κB and autophagy.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
NF-κB signaling mechanisms in HTLV-1-induced adult T-cell leukemia/lymphoma.FEBS J. 2018 Sep;285(18):3324-3336. doi: 10.1111/febs.14492. Epub 2018 May 14. FEBS J. 2018. PMID: 29722927 Free PMC article. Review.
-
Antitumor effects of chloroquine/hydroxychloroquine mediated by inhibition of the NF-κB signaling pathway through abrogation of autophagic p47 degradation in adult T-cell leukemia/lymphoma cells.PLoS One. 2021 Aug 18;16(8):e0256320. doi: 10.1371/journal.pone.0256320. eCollection 2021. PLoS One. 2021. PMID: 34407152 Free PMC article.
-
p53 dysfunction precedes the activation of nuclear factor-κB during disease progression in mice expressing Tax, a human T-cell leukemia virus type 1 oncoprotein.Carcinogenesis. 2013 Sep;34(9):2129-36. doi: 10.1093/carcin/bgt144. Epub 2013 Apr 30. Carcinogenesis. 2013. PMID: 23633516
-
Inducible nitric oxide synthase mediates DNA double strand breaks in Human T-Cell Leukemia Virus Type 1-induced leukemia/lymphoma.Retrovirology. 2015 Aug 12;12:71. doi: 10.1186/s12977-015-0196-y. Retrovirology. 2015. PMID: 26265053 Free PMC article.
-
Mechanisms of persistent NF-kappaB activation by HTLV-I tax.IUBMB Life. 2005 Feb;57(2):83-91. doi: 10.1080/15216540500078715. IUBMB Life. 2005. PMID: 16036567 Review.
Cited by
-
Clinical significance of soluble CADM1 as a novel marker for adult T-cell leukemia/lymphoma.Haematologica. 2021 Feb 1;106(2):532-542. doi: 10.3324/haematol.2019.234096. Haematologica. 2021. PMID: 32054656 Free PMC article.
-
NF-κB and MicroRNA Deregulation Mediated by HTLV-1 Tax and HBZ.Pathogens. 2019 Dec 10;8(4):290. doi: 10.3390/pathogens8040290. Pathogens. 2019. PMID: 31835460 Free PMC article. Review.
-
Trans-homophilic interaction of CADM1 promotes organ infiltration of T-cell lymphoma by adhesion to vascular endothelium.Cancer Sci. 2022 May;113(5):1669-1678. doi: 10.1111/cas.15307. Epub 2022 Mar 8. Cancer Sci. 2022. PMID: 35213073 Free PMC article.
-
Functional and Clinical Characteristics of Cell Adhesion Molecule CADM1 in Cancer.Front Cell Dev Biol. 2021 Jul 30;9:714298. doi: 10.3389/fcell.2021.714298. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34395444 Free PMC article. Review.
-
Autophagy in Viral Development and Progression of Cancer.Front Oncol. 2021 Mar 8;11:603224. doi: 10.3389/fonc.2021.603224. eCollection 2021. Front Oncol. 2021. PMID: 33763351 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

