Striatal Interneurons in Transgenic Nonhuman Primate Model of Huntington's Disease
- PMID: 30837611
- PMCID: PMC6401084
- DOI: 10.1038/s41598-019-40165-w
Striatal Interneurons in Transgenic Nonhuman Primate Model of Huntington's Disease
Abstract
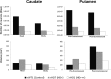
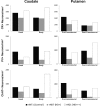
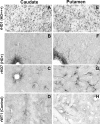
Huntington's disease is an autosomal dominant neurodegenerative disorder associated with progressive motor and cognitive impairments, and the expansion of a cysteine-adenine-guanine trinucleotide (polyglutamine) repeats in exon one of the human huntingtin gene. The pathology of the disease is characterized by a profound degeneration of striatal GABAergic projection neurons with relative sparing of interneurons accompanied with astrogliosis. Here, we describe the striatal pathology in two genotypically different transgenic HD monkeys that exhibit degrees of disease progression that resembled those seen in juvenile- (rHD1) and adult-onset (rHD7) HD. The caudate nucleus and putamen underwent severe neuronal loss in both animals, while the striatal volume was reduced only in rHD1, the most severely affected monkey. The number of GABAergic (calretinin- and parvalbumin-positive) and cholinergic interneurons was also reduced in most striatal regions of these two monkeys, but to variable degrees. Overall, the density of interneurons was increased in rHD1, but not in rHD7, suggesting a relative sparing of interneurons over projection neurons in the striatum of the most affected HD monkey. The neuropil of both the caudate nucleus and putamen was invaded with reactive astrocytes in rHD1, while astrogliosis was much less severe in rHD7 and absent from control. Combined with behavioral data collected from these monkeys, our findings further demonstrate that transgenic HD monkeys share similar disease patterns with HD patients, making them a highly reliable preclinical HD animal model.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Loss-of-Huntingtin in Medial and Lateral Ganglionic Lineages Differentially Disrupts Regional Interneuron and Projection Neuron Subtypes and Promotes Huntington's Disease-Associated Behavioral, Cellular, and Pathological Hallmarks.J Neurosci. 2019 Mar 6;39(10):1892-1909. doi: 10.1523/JNEUROSCI.2443-18.2018. Epub 2019 Jan 9. J Neurosci. 2019. PMID: 30626701 Free PMC article.
-
Amelioration of Huntington's disease phenotype in astrocytes derived from iPSC-derived neural progenitor cells of Huntington's disease monkeys.PLoS One. 2019 Mar 21;14(3):e0214156. doi: 10.1371/journal.pone.0214156. eCollection 2019. PLoS One. 2019. PMID: 30897183 Free PMC article.
-
Plastic and behavioral abnormalities in experimental Huntington's disease: a crucial role for cholinergic interneurons.Neurobiol Dis. 2006 Apr;22(1):143-52. doi: 10.1016/j.nbd.2005.10.009. Epub 2005 Dec 2. Neurobiol Dis. 2006. PMID: 16326108
-
Progress in developing transgenic monkey model for Huntington's disease.J Neural Transm (Vienna). 2018 Mar;125(3):401-417. doi: 10.1007/s00702-017-1803-y. Epub 2017 Nov 10. J Neural Transm (Vienna). 2018. PMID: 29127484 Free PMC article. Review.
-
Disrupted striatal neuron inputs and outputs in Huntington's disease.CNS Neurosci Ther. 2018 Apr;24(4):250-280. doi: 10.1111/cns.12844. CNS Neurosci Ther. 2018. PMID: 29582587 Free PMC article. Review.
Cited by
-
Chromatin accessibility and transcription dynamics during in vitro astrocyte differentiation of Huntington's Disease Monkey pluripotent stem cells.Epigenetics Chromatin. 2019 Nov 13;12(1):67. doi: 10.1186/s13072-019-0313-6. Epigenetics Chromatin. 2019. PMID: 31722751 Free PMC article.
-
Striosomes Mediate Value-Based Learning Vulnerable in Age and a Huntington's Disease Model.Cell. 2020 Nov 12;183(4):918-934.e49. doi: 10.1016/j.cell.2020.09.060. Epub 2020 Oct 27. Cell. 2020. PMID: 33113354 Free PMC article.
-
Liraglutide Improves Cognitive and Neuronal Function in 3-NP Rat Model of Huntington's Disease.Front Pharmacol. 2021 Dec 22;12:731483. doi: 10.3389/fphar.2021.731483. eCollection 2021. Front Pharmacol. 2021. PMID: 35002691 Free PMC article.
-
Large Animal Models of Huntington's Disease: What We Have Learned and Where We Need to Go Next.J Huntingtons Dis. 2020;9(3):201-216. doi: 10.3233/JHD-200425. J Huntingtons Dis. 2020. PMID: 32925082 Free PMC article. Review.
-
Intravenous functional gene transfer throughout the brain of non-human primates using AAV.Res Sq [Preprint]. 2023 Jan 13:rs.3.rs-1370972. doi: 10.21203/rs.3.rs-1370972/v1. Res Sq. 2023. Update in: Nat Nanotechnol. 2023 Oct;18(10):1241-1251. doi: 10.1038/s41565-023-01419-x. PMID: 36789432 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

