Platelet lysate outperforms FCS and human serum for co-culture of primary human macrophages and hMSCs
- PMID: 30837625
- PMCID: PMC6401182
- DOI: 10.1038/s41598-019-40190-9
Platelet lysate outperforms FCS and human serum for co-culture of primary human macrophages and hMSCs
Abstract
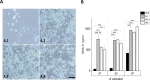
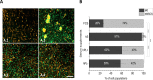
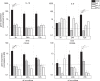
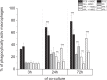
In vitro co-cultures of different primary human cell types are pivotal for the testing and evaluation of biomaterials under conditions that are closer to the human in vivo situation. Especially co-cultures of macrophages and mesenchymal stem cells (MSCs) are of interest, as they are both present and involved in tissue regeneration and inflammatory reactions and play crucial roles in the immediate inflammatory reactions and the onset of regenerative processes, thus reflecting the decisive early phase of biomaterial contact with the host. A co-culture system of these cell types might thus allow for the assessment of the biocompatibility of biomaterials. The establishment of such a co-culture is challenging due to the different in vitro cell culture conditions. For human macrophages, medium is usually supplemented with human serum (hS), whereas hMSC culture is mostly performed using fetal calf serum (FCS), and these conditions are disadvantageous for the respective other cell type. We demonstrate that human platelet lysate (hPL) can replace hS in macrophage cultivation and appears to be the best option for co-cultivation of human macrophages with hMSCs. In contrast to FCS and hS, hPL maintained the phenotype of both cell types, comparable to that of their respective standard culture serum, as well as the percentage of each cell population. Moreover, the expression profile and phagocytosis activity of macrophages was similar to hS.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Gordon, S. & Taylor, P. R. Monocyte and macrophage heterogeneity. Nat Rev Immunol5, 953–964, 10.1038nri1733 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

