Dual role of the Anopheles coluzzii Venus Kinase Receptor in both larval growth and immunity
- PMID: 30837655
- PMCID: PMC6401105
- DOI: 10.1038/s41598-019-40407-x
Dual role of the Anopheles coluzzii Venus Kinase Receptor in both larval growth and immunity
Abstract
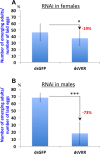
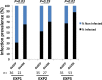
Vector-borne diseases and especially malaria are responsible for more than half million deaths annually. The increase of insecticide resistance in wild populations of Anopheles malaria vectors emphasises the need for novel vector control strategies as well as for identifying novel vector targets. Venus kinase receptors (VKRs) constitute a Receptor Tyrosine Kinase (RTK) family only found in invertebrates. In this study we functionally characterized Anopheles VKR in the Gambiae complex member, Anopheles coluzzii. Results showed that Anopheles VKR can be activated by L-amino acids, with L-arginine as the most potent agonist. VKR was not required for the fecundity of A. coluzzii, in contrast to reports from other insects, but VKR function is required in both Anopheles males and females for development of larval progeny. Anopheles VKR function is also required for protection against infection by Plasmodium parasites, thus identifying a novel linkage between reproduction and immunity in Anopheles. The insect specificity of VKRs as well as the essential function for reproduction and immunity suggest that Anopheles VKR could be a potentially druggable target for novel vector control strategies.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- WHO Fact Sheet, Vector-borned disease, http://www.who.int/mediacentre/factsheets/fs387/en/ (2017).
-
- WHO Malaria Fact Sheet, http://www.who.int/mediacentre/factsheets/fs094/en/ (November 2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

