Influence of CYP2C19 Metabolizer Status on Escitalopram/Citalopram Tolerability and Response in Youth With Anxiety and Depressive Disorders
- PMID: 30837874
- PMCID: PMC6389830
- DOI: 10.3389/fphar.2019.00099
Influence of CYP2C19 Metabolizer Status on Escitalopram/Citalopram Tolerability and Response in Youth With Anxiety and Depressive Disorders
Abstract
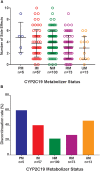
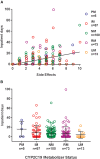
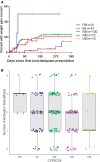
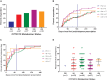
In pediatric patients, the selective serotonin reuptake inhibitors (SSRIs) escitalopram and citalopram (es/citalopram) are commonly prescribed for anxiety and depressive disorders. However, pharmacogenetic studies examining CYP2C19 metabolizer status and es/citalopram treatment outcomes have largely focused on adults. We report a retrospective study of electronic medical record data from 263 youth < 19 years of age with anxiety and/or depressive disorders prescribed escitalopram or citalopram who underwent routine clinical CYP2C19 genotyping. Slower CYP2C19 metabolizers experienced more untoward effects than faster metabolizers (p = 0.015), including activation symptoms (p = 0.029) and had more rapid weight gain (p = 0.018). A larger proportion of slower metabolizers discontinued treatment with es/citalopram than normal metabolizers (p = 0.007). Meanwhile, faster metabolizers responded more quickly to es/citalopram (p = 0.005) and trended toward less time spent in subsequent hospitalizations (p = 0.06). These results highlight a disparity in treatment outcomes with es/citalopram treatment in youth with anxiety and/or depressive disorders when standardized dosing strategies were used without consideration of CYP2C19 metabolizer status. Larger, prospective trials are warranted to assess whether tailored dosing of es/citalopram based on CYP2C19 metabolizer status improves treatment outcomes in this patient population.
Keywords: CYP2C19; SSRI (selective serotonin reuptake inhibitor); antidepressant; anxiety disorders; citalopram; depressive disorder; escitalopram; pharmacogenetics.
Figures
References
-
- Bussing R., Murphy T. K., Storch E. A., McNamara J. P., Reid A. M., Garvan C. W., et al. (2013). Psychometric properties of the Treatment-Emergent Activation and Suicidality Assessment Profile (TEASAP) in youth with OCD. Psychiatry Res. 205 253–261. 10.1016/j.psychres.2012.09.019 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical

