Nanocomposite of Ag-Doped ZnO and AgO Nanocrystals as a Preventive Measure to Control Biofilm Formation in Eggshell and Salmonella spp. Entry Into Eggs
- PMID: 30837963
- PMCID: PMC6389690
- DOI: 10.3389/fmicb.2019.00217
Nanocomposite of Ag-Doped ZnO and AgO Nanocrystals as a Preventive Measure to Control Biofilm Formation in Eggshell and Salmonella spp. Entry Into Eggs
Abstract
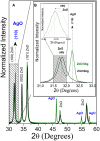
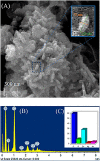
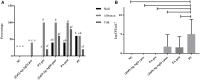
Salmonella spp. is an important foodborne agent of salmonellosis, whose sources in humans often include products of avian origin. The control of this bacterium is difficult especially when Salmonella spp. is organized into biofilms. We hypothesized that the novel nanocomposites of ZnO nanocrystals doped with silver (Ag) and silver oxide (AgO) nanocrystals (ZnO:Ag-AgO) synthesized by the coprecipitation method could control or prevent the formation of Salmonella Enteritidis (SE) and Salmonella Heidelberg (SH) biofilm and its entry into turkey eggs. The diffraction characteristics of ZnO and AgO showed sizes of 28 and 30 nm, respectively. The Zn to Ag substitution into the ZnO crystalline structure was evidenced by the ionic radius of Ag+2 (1.26 Å), which is greater than Zn+2 (0.74 Å). For the SE analyses post-biofilm formation, the ZnO:Ag-AgO was not able to eliminate the biofilm, but the bacterial load was lower than that of the control group. Additionally, SE was able to infiltrate into the eggs and was found in both albumen and yolk. For the SH analyses applied onto the eggshells before biofilm formation, the ZnO:Ag-AgO treatment prevented biofilm formation, and although the bacterium infiltration into the eggs was observed in all treated groups, it was significantly smaller in ZnO:Ag-AgO pre-treated eggs, and SH could not reach the yolk. There was no difference in pore size between groups; therefore, the inhibition of biofilm formation and the prevention of bacterium entry into the egg were attributable to the use of ZnO:Ag-AgO, which was not influenced by the egg structure. Although the amount of Ag and Zn in the shell of the ZnO:Ag-AgO group was greater in relation to the control, this difference was not detected in the other egg components. In the search for new measures that are effective, safe and viable for controlling microorganisms in poultry farming, the application of a nanocomposite of Ag-doped ZnO and AgO nanocrystals appears as an alternative of great potential to prevent Salmonella sp biofilms in eggshells and other surfaces.
Keywords: bacterium; disinfection; nanocrystal; pore; preventive.
Figures


Similar articles
-
Antioxidant, anti-inflammatory, and wound healing effects of topical silver-doped zinc oxide and silver oxide nanocomposites.Int J Pharm. 2022 Apr 5;617:121620. doi: 10.1016/j.ijpharm.2022.121620. Epub 2022 Feb 25. Int J Pharm. 2022. PMID: 35219826
-
Enhancement of antibacterial function by incorporation of silver-doped ZnO nanocrystals onto a laser-induced graphene surface.RSC Adv. 2021 Oct 18;11(54):33883-33889. doi: 10.1039/d1ra06390a. eCollection 2021 Oct 18. RSC Adv. 2021. PMID: 35497311 Free PMC article.
-
Biofilm Formation in Different Salmonella Serotypes Isolated from Poultry.Curr Microbiol. 2019 Jan;76(1):124-129. doi: 10.1007/s00284-018-1599-5. Epub 2018 Dec 17. Curr Microbiol. 2019. PMID: 30560366
-
Mechanisms of egg contamination by Salmonella Enteritidis.FEMS Microbiol Rev. 2009 Jul;33(4):718-38. doi: 10.1111/j.1574-6976.2008.00161.x. Epub 2009 Jan 21. FEMS Microbiol Rev. 2009. PMID: 19207743 Review.
-
Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies.Food Res Int. 2019 May;119:530-540. doi: 10.1016/j.foodres.2017.11.024. Epub 2017 Nov 21. Food Res Int. 2019. PMID: 30884686 Review.
Cited by
-
Multifunctional phototheranostic agent ZnO@Ag for anti-infection through photothermal/photodynamic therapy.Front Chem. 2022 Nov 9;10:1054739. doi: 10.3389/fchem.2022.1054739. eCollection 2022. Front Chem. 2022. PMID: 36438866 Free PMC article.
-
Toxicity Assessment of New Ag-ZnO/AgO Nanocomposites: An In Vitro and In Vivo Approach.J Funct Biomater. 2024 Feb 20;15(3):51. doi: 10.3390/jfb15030051. J Funct Biomater. 2024. PMID: 38535244 Free PMC article.
-
Hybrid Pectin-Liposome Formulation against Multi-Resistant Bacterial Strains.Pharmaceutics. 2020 Aug 14;12(8):769. doi: 10.3390/pharmaceutics12080769. Pharmaceutics. 2020. PMID: 32823823 Free PMC article.
-
Utilization of Antibacterial Nanoparticles in Photocurable Additive Manufacturing of Advanced Composites for Improved Public Health.Polymers (Basel). 2021 Aug 6;13(16):2616. doi: 10.3390/polym13162616. Polymers (Basel). 2021. PMID: 34451156 Free PMC article.
-
Application of ZnO Nanocrystals as a Surface-Enhancer FTIR for Glyphosate Detection.Nanomaterials (Basel). 2021 Feb 17;11(2):509. doi: 10.3390/nano11020509. Nanomaterials (Basel). 2021. PMID: 33671396 Free PMC article.
References
-
- BRASIL (2008). Resolução no 91 De 28 de Novembro de 2008. Agência Nacional de Vigilância Sanitária, Ministério da Saúde. Diário official da União. Brasilia 1 de dezembro de 2008. Available in: http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2008/res0091_28_11_2008....
-
- Cardoso A. L. S. P., Tessari E. N. C., Castro A. G. M., Kanashiro A. M. I., Gama N. M. S. Q. (2001). Pesquisa de coliformes totais e coliformes fecais analisados em ovos comerciais no laboratório de patologia avícola de descalvado. Arq. Inst. Bio. 68 19–22.
LinkOut - more resources
Full Text Sources
Miscellaneous

