BCG Vaccination Induces M. avium and M. abscessus Cross-Protective Immunity
- PMID: 30837992
- PMCID: PMC6389677
- DOI: 10.3389/fimmu.2019.00234
BCG Vaccination Induces M. avium and M. abscessus Cross-Protective Immunity
Abstract
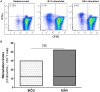
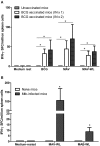
Pulmonary non-tuberculous mycobacterial (NTM) infections particularly caused by Mycobacterium avium complex (MAC) and Mycobacterium abscessus (MAB) are becoming major health problems in the U.S. New therapies or vaccines which will help prevent the disease, shorten treatment duration and/or increase treatment success rates are urgently needed. This study was conducted with the objective of testing the hypothesis that Bacillus Calmette Guerin (BCG), a vaccine used for prevention of serious forms of tuberculosis (TB) in children and adolescents in tuberculosis hyperendemic countries, induces cross-protective T cell immunity against Mycobacterium avium (MAV) and MAB. Human TB and NTM cross-protective T cells were quantified using flow cytometric assays. The ability of BCG expanded T cells to inhibit the intracellular growth of MAV and MAB was assessed in co-cultures with infected autologous macrophages. In both BCG-vaccinated and M. tuberculosis (Mtb)-infected mice, NTM cross-reactive immunity was measured using IFN-γ ELISPOT assays. Our results demonstrate the following key findings: (i) peripheral blood mononuclear cells from TB skin test-positive individuals contain MAV and MAB cross-reactive T cells, (ii) both BCG vaccination and Mtb infection of mice induce MAV and MAB cross-reactive splenic cells, (iii) BCG-expanded T cells inhibit intracellular MAV and MAB, (iv) CD4, CD8, and γδ T cells play important roles in inhibition of intracellular MAV and MAB and (v) BCG vaccination of healthy volunteers induces TB and NTM cross-reactive T cells. In conclusion, BCG-vaccination induces NTM cross-reactive immunity, and has the potential for use as a vaccine or immunotherapy to prevent and/or treat pulmonary NTM disease.
Keywords: BCG; abscessus; avium; mycobacteria; nontuberculous.
Figures

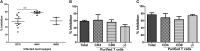

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

