Beta2-Integrins and Interacting Proteins in Leukocyte Trafficking, Immune Suppression, and Immunodeficiency Disease
- PMID: 30837997
- PMCID: PMC6389632
- DOI: 10.3389/fimmu.2019.00254
Beta2-Integrins and Interacting Proteins in Leukocyte Trafficking, Immune Suppression, and Immunodeficiency Disease
Abstract
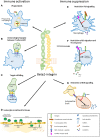
Beta2-integrins are complex leukocyte-specific adhesion molecules that are essential for leukocyte (e.g., neutrophil, lymphocyte) trafficking, as well as for other immunological processes such as neutrophil phagocytosis and ROS production, and T cell activation. Intriguingly, however, they have also been found to negatively regulate cytokine responses, maturation, and migratory responses in myeloid cells such as macrophages and dendritic cells, revealing new, and unexpected roles of these molecules in immunity. Because of their essential role in leukocyte function, a lack of expression or function of beta2-integrins causes rare immunodeficiency syndromes, Leukocyte adhesion deficiency type I, and type III (LAD-I and LAD-III). LAD-I is caused by reduced or lost expression of beta2-integrins, whilst in LAD-III, beta2-integrins are expressed but dysfunctional because a major integrin cytoplasmic regulator, kindlin-3, is mutated. Interestingly, some LAD-related phenotypes such as periodontitis have recently been shown to be due to an uncontrolled inflammatory response rather than to an uncontrolled infection, as was previously thought. This review will focus on the recent advances concerning the regulation and functions of beta2-integrins in leukocyte trafficking, immune suppression, and immune deficiency disease.
Keywords: integrin; kindlin-3; leukocyte adhesion cascade; leukocyte adhesion deficiency; trafficking.
Figures
References
-
- Rothlein R, Dustin ML, Marlin SD, Springer TA. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. (1986) 137:1270–4. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

