Carbon Acidity in Enzyme Active Sites
- PMID: 30838206
- PMCID: PMC6389717
- DOI: 10.3389/fbioe.2019.00025
Carbon Acidity in Enzyme Active Sites
Abstract
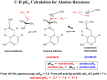
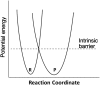
The pKa values for substrates acting as carbon acids (i.e., C-H deprotonation reactions) in several enzyme active sites are presented. The information needed to calculate them includes the pKa of the active site acid/base catalyst and the equilibrium constant for the deprotonation step. Carbon acidity is obtained from the relation pKeq = p -p = ΔpKa for a proton transfer reaction. Five enzymatic free energy profiles (FEPs) were calculated to obtain the equilibrium constants for proton transfer from carbon in the active site, and six additional proton transfer equilibrium constants were extracted from data available in the literature, allowing substrate C-H pKas to be calculated for 11 enzymes. Active site-bound substrate C-H pKa values range from 5.6 for ketosteroid isomerase to 16 for proline racemase. Compared to values in water, enzymes lower substrate C-H pKas by up to 23 units, corresponding to 31 kcal/mol of carbanion stabilization energy. Calculation of Marcus intrinsic barriers (Δ ) for pairs of non-enzymatic/enzymatic reactions shows significant reductions in Δ for cofactor-independent enzymes, while pyridoxal phosphate dependent enzymes appear to increase Δ to a small extent as a consequence of carbanion resonance stabilization. The large increases in carbon acidity found here are central to the large rate enhancements observed in enzymes that catalyze carbon deprotonation.
Keywords: PKA; carbanion stability; carbon acid; enzyme; general acid/base catalysis; marcus theory; pyridoxal phosphate.
Figures



References
-
- Alberty R. A., Peirce W. H. (1957). Studies of the enzyme fumarase. V.1 calculation of minimum and maximum values of constants for the general fumarase mechanism2. J. Am. Chem. Soc. 79, 1526–1530. 10.1021/ja01564a002 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

