A Primer on AmpC β-Lactamases: Necessary Knowledge for an Increasingly Multidrug-resistant World
- PMID: 30838380
- PMCID: PMC6763639
- DOI: 10.1093/cid/ciz173
A Primer on AmpC β-Lactamases: Necessary Knowledge for an Increasingly Multidrug-resistant World
Abstract
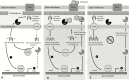
Understanding the nuances of AmpC β-lactamase-mediated resistance can be challenging, even for the infectious diseases specialist. AmpC resistance can be classified into 3 categories: (1) inducible chromosomal resistance that emerges in the setting of a β-lactam compound, (2) stable derepression due to mutations in ampC regulatory genes, or (3) the presence of plasmid-mediated ampC genes. This review will mainly focus on inducible AmpC resistance in Enterobacteriaceae. Although several observational studies have explored optimal treatment for AmpC producers, few provide reliable insights into effective management approaches. Heterogeneity within the data and inherent selection bias make inferences on effective β-lactam choices problematic. Most experts agree it is prudent to avoid expanded-spectrum (ie, third-generation) cephalosporins for the treatment of organisms posing the greatest risk of ampC induction, which has best been described in the context of Enterobacter cloacae infections. The role of other broad-spectrum β-lactams and the likelihood of ampC induction by other Enterobacteriaceae are less clear. We will review the mechanisms of resistance and triggers resulting in AmpC expression, the species-specific epidemiology of AmpC production, approaches to the detection of AmpC production, and treatment options for AmpC-producing infections.
Keywords: Citrobacter freundii; Enterobacter cloacae; Serratia marcescens; antimicrobial resistance.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Species-specific Epidemiology of AmpC Production.Clin Infect Dis. 2020 Dec 17;71(10):2767-2768. doi: 10.1093/cid/ciaa120. Clin Infect Dis. 2020. PMID: 32022849 No abstract available.

