Tubule-vascular feedback in renal autoregulation
- PMID: 30838873
- PMCID: PMC6620598
- DOI: 10.1152/ajprenal.00381.2018
Tubule-vascular feedback in renal autoregulation
Abstract
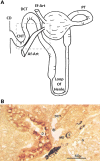
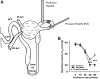
Afferent arteriole (Af-Art) diameter regulates pressure and flow into the glomerulus, which are the main determinants of the glomerular filtration rate. Thus, Af-Art resistance is crucial for Na+ filtration. Af-Arts play a role as integrative centers, where systemic and local systems interact to determine the final degree of resistance. The tubule of a single nephron contacts an Af-Art of the same nephron at two locations: in the transition of the thick ascending limb to the distal tubule (macula densa) and again in the connecting tubule. These two sites are the anatomic basis of two intrinsic feedback mechanisms: tubule-glomerular feedback and connecting tubule-glomerular feedback. The cross communications between the tubules and Af-Arts integrate tubular Na+ and water processing with the hemodynamic conditions of the kidneys. Tubule-glomerular feedback provides negative feedback that tends to avoid salt loss, and connecting tubule-glomerular feedback provides positive feedback that favors salt excretion by modulating tubule-glomerular feedback (resetting it) and increasing glomerular filtration rate. These feedback mechanisms are also exposed to systemic modulators (hormones and the nervous system); however, they can work in isolated kidneys or nephrons. The exaggerated activation or absence of any of these mechanisms may lead to disequilibrium in salt and water homeostasis, especially in extreme conditions (e.g., high-salt diet/low-salt diet) and may be part of the pathogenesis of some diseases. In this review, we focus on molecular signaling, feedback interactions, and the physiological roles of these two feedback mechanisms.
Keywords: connecting tubule-glomerular feedback; epithelial sodium channel; macula densa; renal autoregulation; sodium-potassium-two chloride cotransporter; tubule-glomerular feedback.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

