Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease
- PMID: 30839102
- PMCID: PMC6402279
- DOI: 10.1002/14651858.CD012930.pub2
Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a respiratory condition causing accumulation of mucus in the airways, cough, and breathlessness; the disease is progressive and is the fourth most common cause of death worldwide. Current treatment strategies for COPD are multi-modal and aim to reduce morbidity and mortality and increase patients' quality of life by slowing disease progression and preventing exacerbations. Fixed-dose combinations (FDCs) of a long-acting beta2-agonist (LABA) plus a long-acting muscarinic antagonist (LAMA) delivered via a single inhaler are approved by regulatory authorities in the USA, Europe, and Japan for the treatment of COPD. Several LABA/LAMA FDCs are available and recent meta-analyses have clarified their utility versus their mono-components in COPD. Evaluation of the efficacy and safety of once-daily LABA/LAMA FDCs versus placebo will facilitate the comparison of different FDCs in future network meta-analyses.
Objectives: We assessed the evidence for once-daily LABA/LAMA combinations (delivered in a single inhaler) versus placebo on clinically meaningful outcomes in patients with stable COPD.
Search methods: We identified trials from Cochrane Airways' Specialised Register (CASR) and also conducted a search of the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch). We searched CASR and trial registries from their inception to 3 December 2018; we imposed no restriction on language of publication.
Selection criteria: We included parallel-group and cross-over randomised controlled trials (RCTs) comparing once-daily LABA/LAMA FDC versus placebo. We included studies reported as full-text, those published as abstract only, and unpublished data. We excluded very short-term trials with a duration of less than 3 weeks. We included adults (≥ 40 years old) with a diagnosis of stable COPD. We included studies that allowed participants to continue using their ICS during the trial as long as the ICS was not part of the randomised treatment.
Data collection and analysis: Two review authors independently screened the search results to determine included studies, extracted data on prespecified outcomes of interest, and assessed the risk of bias of included studies; we resolved disagreements by discussion with a third review author. Where possible, we used a random-effects model to meta-analyse extracted data. We rated all outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system and presented results in 'Summary of findings' tables.
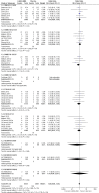
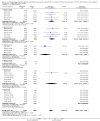
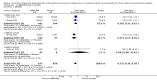
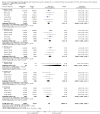
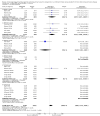
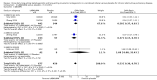
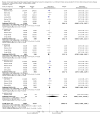
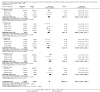
Main results: We identified and included 22 RCTs randomly assigning 8641 people with COPD to either once-daily LABA/LAMA FDC (6252 participants) or placebo (3819 participants); nine studies had a cross-over design. Studies had a duration of between three and 52 weeks (median 12 weeks). The mean age of participants across the included studies ranged from 59 to 65 years and in 21 of 22 studies, participants had GOLD stage II or III COPD. Concomitant inhaled corticosteroid (ICS) use was permitted in all of the included studies (where stated); across the included studies, between 28% to 58% of participants were using ICS at baseline. Six studies evaluated the once-daily combination of IND/GLY (110/50 μg), seven studies evaluated TIO/OLO (2.5/5 or 5/5 μg), eight studies evaluated UMEC/VI (62.5/5, 125/25 or 500/25 μg) and one study evaluated ACD/FOR (200/6, 200/12 or 200/18 μg); all LABA/LAMA combinations were compared with placebo.The risk of bias was generally considered to be low or unknown (insufficient detail provided), with only one study per domain considered to have a high risk of bias except for the domain 'other bias' which was determined to be at high risk of bias in four studies (in three studies, disease severity was greater at baseline in participants receiving LABA/LAMA compared with participants receiving placebo, which would be expected to shift the treatment effect in favour of placebo).Compared to the placebo, the pooled results for the primary outcomes for the once-daily LABA/LAMA arm were as follows: all-cause mortality, OR 1.88 (95% CI 0.81 to 4.36, low-certainty evidence); all-cause serious adverse events (SAEs), OR 1.06 (95% CI 0.88 to 1.28, high-certainty evidence); acute exacerbations of COPD (AECOPD), OR 0.53 (95% CI 0.36 to 0.78, moderate-certainty evidence); adjusted St George's Respiratory Questionnaire (SGRQ) score, MD -4.08 (95% CI -4.80 to -3.36, high-certainty evidence); proportion of SGRQ responders, OR 1.75 (95% CI 1.54 to 1.99). Compared with placebo, the pooled results for the secondary outcomes for the once-daily LABA/LAMA arm were as follows: adjusted trough forced expiratory volume in one second (FEV1), MD 0.20 L (95% CI 0.19 to 0.21, moderate-certainty evidence); adjusted peak FEV1, MD 0.31 L (95% CI 0.29 to 0.32, moderate-certainty evidence); and all-cause AEs, OR 0.95 (95% CI 0.86 to 1.04; high-certainty evidence). No studies reported data for the 6-minute walk test. The results were generally consistent across subgroups for different LABA/LAMA combinations and doses.
Authors' conclusions: Compared with placebo, once-daily LABA/LAMA (either IND/GLY, UMEC/VI or TIO/OLO) via a combination inhaler is associated with a clinically significant improvement in lung function and health-related quality of life in patients with mild-to-moderate COPD; UMEC/VI appears to reduce the rate of exacerbations in this population. These conclusions are supported by moderate or high certainty evidence based on studies with an observation period of up to one year.
Conflict of interest statement
U Maqsood: none known.
D Evans: provides freelance writing support to medical communications agencies.
T Ho: none known.
K Palmer: none known.
F Eccles: none known.
Ran Wang: none known.
M Munavvar: Received honorarium for lectures and to attend the European Respiratory Society meeting, in the past, from various companies, including Astra Zeneca, Chiesi, GSK, Almirall, Boehringer Ingelheim, and Pneumrx.
I Crossingham: none known.
Figures






































Update of
- doi: 10.1002/14651858.CD012930
References
References to studies included in this review
Bateman 2013 {published data only}
-
- Asai K, Hirata K, Hashimoto S, Fukuchi Y, Kitawaki T, Ikeda K, et al. Efficacy and safety of indacaterol/glycopyrronium in Japanese patients with COPD: pooled analysis of SHINE and ARISE. Respiratory Investigation 2016;54(6):428‐35. [2212‐5353] - PubMed
-
- Barnes N, Bateman E, Gallagher N, Green Y, Horton R, Henley M, et al. QVA149 once daily provides superior bronchodilation versus indacaterol, glycopyrronium, tiotropium and placebo: the SHINE study. Thorax 2012; Vol. 67:A147 [P192].
-
- Bateman E, Ferguson GT, Barnes N, Gallagher N, Green Y, Horton R, et al. Benefits of dual bronchodilation with QVA149 once daily versus placebo, indacaterol, NVA237 and tiotropium in patients with COPD: the SHINE study. European Respiratory Journal. 2012; Vol. 40:509s [2810].
-
- Bateman ED, Welte T, Hashimoto S, Gallagher N, Green Y, Horton R, et al. Dual bronchodilation with once‐daily QVA149 provides superior bronchodilation compared to its mono‐components and tiotropium in all subgroups of patients with COPD: the shine study. American Journal of Respiratory and Critical Care Medicine 2013;187:A4263.
Beeh 2014 {published data only}
-
- Beeh KM, Korn S, Beier J, Jadayel D, Henley M, D'Andrea P, et al. Effect of QVA149 on lung volumes and exercise tolerance in COPD patients: the BRIGHT study. Respiratory Medicine 2014;108(4):584‐92. - PubMed
-
- Beeh KM, Korn S, Beier J, Jadayel D, Henley M, Tylek J, et al. QVA149 once daily improves exercise tolerance and lung function in patients with moderate to severe COPD: the BRIGHT study. Thorax 2012;67:A147 [P191].
-
- Beeh KM, Korn S, Beier J, Jadayel D, Henley M, Tylek J, et al. QVA149 once daily reduces lung hyperinflation, improves lung function and volumes as measured by body plethysomography: the BRIGHT study. American Journal of Respiratory and Critical Care Medicine 2013;187:A4259.
Beeh 2015 {published data only}
-
- Beeh K, Westerman J, Kirsten A, Hebert J, Gronke L, Hamilton A, et al. The 24‐h lung‐function profile of once‐daily tiotropium and olodaterol fixed‐dose combination in chronic obstructive pulmonary disease. Pulmonary Pharmacology and Therapeutics 2015;32:53‐9. [1094‐5539] - PubMed
-
- Derom E, Westerman J, Groenke L, Hamilton A, Li C, Beeh K. The 24‐hour lung function profile of once‐daily tiotropium and olodaterol fixed‐dose combination compared with placebo and monotherapies in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2014;69:A190‐1.
-
- NCT01559116. Characterization of 24‐hour lung function profiles of inhaled tiotropium + olodaterol fixed dose combination in patients suffering from chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT01559116 (first received 21 March 2012).
Celli 2014 {published data only}
-
- Celli B, Crater G, Kilbride S, Mehta R, Tabberer M, Kalberg C, et al. Once‐daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest 2014;145(5):981‐91. - PubMed
-
- Celli BR, Crater G, Kilbride S, Mehta R, Tabberer M, Kalberg CJ, et al. A 24‐week randomized, double‐blind, placebo‐controlled study of the efficacy and safety of once‐daily umeclidinium/vilanterol 125/25 mcg in COPD. American Journal of Respiratory and Critical Care Medicine 2013;187:A2435.
-
- Donohue JF, Niewoehner D, Brooks J, O'Dell D, Church A. Safety and tolerability of once‐daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52‐week, randomized, double‐blind, placebo‐controlled study. Respiratory Research 2014;15:78. - PMC - PubMed
-
- NCT01313637. A 24‐week evaluation of GSK573719/Vilanterol (125/25mcg) and components in COPD. clinicaltrials.gov/ct2/show/NCT01313637 (first received 14 March 2011).
Dahl 2013 {published data only}
-
- Dahl R, Chapman K, Rudolf M, Mehta R, Kho P, Alagappan V, et al. QVA149 administered once daily provides significant improvements in lung function over 1 year in patients with COPD: the ENLIGHTEN study. European Respiratory Journal 2012;40:529s [P2896].
-
- Dahl R, Chapman K, Rudolf M, Mehta R, Kho P, Alagappan V, et al. QVA149 once daily provides significant improvements in lung function over 1 year in patients with COPD: the Enlighten study. Thorax 2012;67:A146 [P190].
-
- Dahl R, Chapman KR, Rudolf M, Mehta R, Kho P, Alagappan VK, et al. Safety and efficacy of dual bronchodilation with QVA149 in COPD patients: the ENLIGHTEN study. Respiratory Medicine 2013;107(10):1558‐67. - PubMed
-
- Dahl R, Jadayel D, Alagappan V, Chen H, Banerji D. Once‐daily QVA149 provides the same efficacy as the free combination of its monocomponents indacaterol and glycopyrronium: the BEACON study. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42:694s [P3390].
-
- NCT01120717. A study to assess the long‐term safety of QVA149 (ENLIGHTEN). clinicaltrials.gov/ct2/show/NCT01120717 (first received 11 May 2010).
Donohue 2013 {published data only}
-
- Donohue JF, Maleki‐Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once‐daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respiratory Medicine 2013;107(10):1538‐46. - PubMed
-
- NCT01313650. A 24‐week evaluation of GSK573719/Vilanterol (62.5/25mcg) and components in COPD (DB2113373). clinicaltrials.gov/ct2/show/NCT01313650 (first received 14 March 2011).
Feldman 2012 {published data only}
-
- Feldman G, Walker RR, Brooks J, Mehta R, Crater G. 28‐day safety and tolerability of umeclidinium in combination with vilanterol in COPD: a randomized placebo‐controlled trial. Pulmonary Pharmacology and Therapeutics 2012;25(6):465‐71. - PubMed
Larbig 2015 {published data only}
-
- Larbig M, Fowler‐taylor A, Maitra S, Schubert‐Tennigkeit A, Banerji D. Efficacy and safety of IND/GLY versus placebo and tiotropium in symptomatic patients with moderate‐to‐severe COPD: the 52‐week radiate study. Respirology 2015;20:44. [1323‐7799]
-
- NCT01610037. Comparison of long‐term safety of the combination product QVA149A against placebo and standard of care treatment in chronic obstructive pulmonary disease patients with moderate to severe airflow limitation. clinicaltrials.gov/ct2/show/NCT01610037 (first received 1 June 2012).
Mahler 2014 {published data only}
-
- D'Urzo A, Mahler D, Worth H, White T, Alagappan V, Chen H, et al. Patients with severe COPD show significant improvements in dyspnea and lung function with once‐daily QVA149: the blaze study. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2014; Vol. 145.
-
- D'Urzo A, Mahler DA, Decramer M, Worth H, White T, Alagappan VKT, et al. Superior lung function with once‐daily QVA149 translates into improvements in patient‐reported breathlessness compared with placebo and tiotropium in COPD patients: the BLAZE study. Thorax. 2013:A184 [P236].
-
- Mahler DA, Decramer M, D'Urzo A, Worth H, White T, Alagappan VKT, et al. Dual bronchodilation with QVA149 reduces patient‐reported dyspnoea in COPD: the BLAZE study. European Respiratory Journal 2014;43(6):1599‐609. - PubMed
-
- Mahler DA, Decramer M, D'Urzo AD, Worth H, White T, Alagappan V, et al. Superior lung function with once‐daily QVA149 translates into improvements in patient‐reported breathlessness compared with placebo and tiotropium in COPD patients: the BLAZE study. American Journal of Respiratory and Critical Care Medicine 2013;187:A6070.
Maltais 2014 {published data only}
-
- Maltais F, Galdiz Iturri JB, Kirsten A, Singh D, Hamilton A, Tetzlaff K, et al. Effects of 12 weeks of once‐daily tiotropium and olodaterol fixed‐dose combination on exercise endurance in patients with COPD. European Respiratory Journal 2014;44:P283.
-
- Maltais F, Galdiz Iturri JB, Kirsten A, Singh D, Hamilton A, Tetzlaff K, et al. Effects of 12 weeks of once‐daily tiotropium and olodaterol fixed‐dose combination on exercise endurance in patients with COPD. Thorax 2014;69:A186‐7.
-
- Maltais F, O'Donnell D, Gáldiz Iturri JB, Kirsten AM, Singh D, Hamilton A, et al. Effect of 12 weeks of once‐daily tiotropium/olodaterol on exercise endurance during constant work‐rate cycling and endurance shuttle walking in chronic obstructive pulmonary disease. Therapeutic Advances in Respiratory Disease 2018;12:1753465818755091. [DOI: 10.1177/1753465818755091] - DOI - PMC - PubMed
-
- NCT01525615. A study to determine the effect of tiotropium + olodaterol fixed dose combination on exercise endurance time during constant work rate cycle ergometry test in COPD. clinicaltrials.gov/ct2/show/NCT01525615 (first received 3 February 2012).
Maltais 2014b {published data only}
-
- Maltais F, Singh S, Donald A, Church A, Crater G, Goh A, et al. Effects of a combination of vilanterol and umeclidinium on exercise endurance in subjects with COPD: two randomised clinical trials. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. Vol. 42:145s [P761].
-
- Maltais F, Singh S, Donald A, Crater G, Church A, Goh A. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: Two randomized, double‐blind clinical trials. Therapeutic Advances in Respiratory Disease 2016;10(3):Erratum in: Therapeutic Advances in Respiratory Disease 2014; 8: 169‐181; 289. [1753‐4666] - PubMed
-
- Maltais F, Singh S, Donald AC, Crater G, Church A, Goh AH, et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double‐blind clinical trials. Therapeutic Advances in Respiratory Disease 2014;8(6):169‐81. [1753‐4666] - PubMed
-
- Singh S, Maltais F, Tombs L, Fahy WA, Vahdati‐Bolouri M, Locantore N, et al. Relationship between exercise endurance and static hyperinflation in a post hoc analysis of two clinical trials in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:203‐15. - PMC - PubMed
Maltais 2014c {published data only}
-
- Maltais F, Singh S, Donald A, Church A, Crater G, Goh A, et al. Effects of a combination of vilanterol and umeclidinium on exercise endurance in subjects with COPD: two randomised clinical trials. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42:145s [P761].
-
- Maltais F, Singh S, Donald A, Crater G, Church A, Goh A. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double‐blind clinical trials. Therapeutic Advances in Respiratory Disease 2016;10(3):Erratum in: Therapeutic Advances in Respiratory Disease 2014; 8: 169‐181; 289. [1753‐4666] - PubMed
-
- Maltais F, Singh S, Donald AC, Crater G, Church A, Goh AH, et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomized, double‐blind clinical trials. Therapeutic Advances in Respiratory Disease 2014;8(6):169‐81. [1753‐4666] - PubMed
-
- Singh S, Maltais F, Tombs L, Fahy WA, Vahdati‐Bolouri M, Locantore N, et al. Relationship between exercise endurance and static hyperinflation in a post hoc analysis of two clinical trials in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:203‐15. - PMC - PubMed
NCT00626522 {published data only}
-
- NCT00626522. Aclidinium/formoterol fixed combination dose finding study. clinicaltrials.gov/ct2/show/NCT00626522 (first received 29 February 2008).
NCT02275052 {published data only}
-
- NCT02275052. A randomized, double‐blind, placebo‐controlled evaluation of the effect of the combination of umeclidinium and vilanterol on exercise endurance time in subjects with COPD. clinicaltrials.gov/ct2/show/NCT02275052 (first received 27 October 2014).
O'Donnell 2015a {published data only}
-
- NCT01533922. Effect on exercise endurance and lung hyperinflation of tiotropium + olodaterol in COPD patients. clinicaltrials.gov/ct2/show/NCT01533922 (first received 16 February 2012).
-
- NCT01533935. Effect on exercise endurance and lung hyperinflation of tiotropium + olodaterol in COPD patients. clinicaltrials.gov/ct2/show/NCT01533935 (first received 16 February 2012).
-
- O'Donnell D, Casaburi R, Sousa D, Xue W, Frith P, Hamilton A, et al. Effects of 6 weeks' treatment with once‐daily tiotropium and olodaterol fixed‐dose combination on inspiratory capacity and exercise endurance in patients with COPD: the MORACTOTM studies. American Journal of Respiratory and Critical Care Medicine 2015;191:A3972.
O'Donnell 2015b {published data only}
-
- NCT01533922. Effect on exercise endurance and lung hyperinflation of tiotropium + olodaterol in COPD patients. clinicaltrials.gov/ct2/show/NCT01533922 (first received 16 February 2012).
-
- NCT01533935. Effect on exercise endurance and lung hyperinflation of tiotropium + olodaterol in COPD patients. clinicaltrials.gov/ct2/show/NCT01533935 (first received 16 February 2012).
-
- O'Donnell D, Casaburi R, Sousa D, Xue W, Frith P, Hamilton A, et al. Effects of 6 weeks' treatment with once‐daily tiotropium and olodaterol fixed‐dose combination on inspiratory capacity and exercise endurance in patients with COPD: the MORACTOTM studies. American Journal of Respiratory and Critical Care Medicine 2015;191:A3972.
Siler 2016 {published data only}
-
- NCT02152605. A phase IIIb study to evaluate the efficacy of umeclidinium/vilanterol (UMEC/VI) in subjects with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT02152605 (first received 2 June 2014).
-
- Siler TM, Donald AC, O'Dell D, Church A, Fahy WA. A randomized, parallel‐group study to evaluate the efficacy of umeclidinium/vilanterol 62.5/25 mug on health‐related quality of life in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2016;11(1):971‐9. [1178‐2005] - PMC - PubMed
Singh 2016a {published data only}
-
- NCT01964352. Tiotropium + olodaterol fixed dose combination (FDC) in chronic obstructive pulmonary disease (OTEMTO 1). clinicaltrials.gov/ct2/show/NCT01964352 (first received 17 October 2013).
-
- NCT02006732. Tiotropium + olodaterol fixed dose combination (FDC) in chronic obstructive pulmonary disease (OTEMTO 2). clinicaltrials.gov/ct2/show/NCT02006732 (first received 10 December 2013).
-
- Singh D, Ferguson GT, Bolitschek J, Gronke L, Hallmann C, Bennett N, et al. Tiotropium + olodaterol fixed‐dose combination shows clinically meaningful improvements in quality of life versus placebo. European Respiratory Journal 2015;46:PA2958. [DOI: 10.1183/13993003.congress-2015.PA2958] - DOI - PubMed
-
- Singh D, Ferguson GT, Bolitschek J, Gronke L, Hallmann C, Bennett N, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respiratory Medicine 2015;109(10):1312‐9. - PubMed
-
- Singh D, Ferguson GT, Bolitschek J, Grönke L, Hallmann C, Bennett N, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life versus placebo. Primary Care Respiratory Medicine 2016;26:21‐CR050. - PubMed
Singh 2016b {published data only}
-
- NCT01964352. Tiotropium + olodaterol fixed dose combination (FDC) in chronic obstructive pulmonary disease (OTEMTO 1). clinicaltrials.gov/ct2/show/NCT01964352 (first received 17 October 2013).
-
- NCT02006732. Tiotropium+olodaterol fixed dose combination (FDC) in chronic obstructive pulmonary disease (OTEMTO 2). clinicaltrials.gov/ct2/show/NCT02006732 (first received 10 December 2013).
-
- Singh D, Ferguson GT, Bolitschek J, Gronke L, Hallmann C, Bennett N, et al. Tiotropium + olodaterol fixed‐dose combination shows clinically meaningful improvements in quality of life versus placebo. European Respiratory Journal 2015;46:PA2958. [DOI: ] - PubMed
-
- Singh D, Ferguson GT, Bolitschek J, Gronke L, Hallmann C, Bennett N, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respiratory Medicine 2015;109(10):1312‐9. - PubMed
-
- Singh D, Ferguson GT, Bolitschek J, Grönke L, Hallmann C, Bennett N, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life versus placebo. Primary Care Respiratory Medicine 2016;26(16022):21‐CR050. [ES:2055‐1010: IL:2055‐1010] - PubMed
Troosters 2016 {published data only}
-
- Lavoie K, Sedeno M, Li P, Troosters T, Hamilton A, Sousa D, et al. Effects of bronchodilator therapy and exercise with self‐management behaviour‐modification on psychological and cognitive outcomes in COPD. European Respiratory Journal 2017;50:OA4669.
-
- NCT02085161. To evaluate the effect of inhaled medication together with exercise and activity training on exercise capacity and daily activities in patients with chronic lung disease with obstruction of airways. clinicaltrials.gov/ct2/show/NCT02085161 (first received 12 March 2014).
-
- Troosters T, Bourbeau J, Maltais F, Leidy N, Erzen D, Sousa D, et al. Effect of 8 and 12 weeks' once‐daily tiotropium and olodaterol, alone and combined with exercise training, on exercise endurance during walking in patients with COPD. Pneumologie 2017;71(1):159‐60.
-
- Troosters T, Bourbeau J, Maltais F, Leidy N, Erzen D, Sousa D, et al. Enhancing exercise tolerance and physical activity in COPD with combined pharmacological and non‐pharmacological interventions: PHYSACTO randomised, placebo‐controlled study design. BMJ Open 2016;6(4):e010106. [2044‐6055] - PMC - PubMed
Watz 2016 {published data only}
-
- Watz H, Mailaender C, Kirsten AM. Effects of indacaterol/glycopyrronium on lung function and physical activity in patients with moderate to severe COPD. Thorax 2015;70:A146‐7.
Zheng 2014 {published data only}
-
- Zheng J, Zhong N, Newlands A, Church A, Goh AH. Efficacy and safety of once‐daily inhaled umeclidinium/vilanterol in Asian patients with COPD: results from a randomized, placebo‐controlled study. International Journal of Chronic Obstructive Pulmonary Disease 2015;10(1):1753‐67. [1178‐2005] - PMC - PubMed
-
- Zheng JP, Newlands AH, Church A, Goh AH. The efficacy and safety of inhaled umeclidinium bromide/vilanterol in Asian patients with chronic obstructive pulmonary disease. Respirology 2014;19:22.
References to studies excluded from this review
Aalbers 2015 {published data only}
-
- Aalbers R, Maleki‐Yazdi M, Hamilton A, Waitere‐Wijker S, Zhao Y, Amatto V, et al. Randomized, double‐blind, dose‐finding study for tiotropium when added to olodaterol, administered via the Respimat(R) inhaler in patients with chronic obstructive pulmonary disease. Advances in Therapy 2015;32(9):809‐22. [0741‐238X] - PMC - PubMed
-
- Aalbers R, Reza Maleki‐Yazdi M, Hamilton A, Waitere‐Wijker S, Pivovarova A, Schmidt O, et al. Dose‐finding study for tiotropium and olodaterol when administered in combination via the Respimat inhaler in patients with COPD [Abstract]. European Respiratory Journal 2012;40:525s [P2882].
-
- NCT01040403. Determining optimal free dose combination of tiotropium bromide and BI 1744 CL in chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT01040403 (first received 29 December 2009).
Asai 2013 {published data only}
-
- Asai K, Hirata K, Hashimoto S, Fukuchi Y, Kitawaki T, Ikeda K, et al. Efficacy and safety of indacaterol/glycopyrronium in Japanese patients with COPD: pooled analysis of SHINE and ARISE. Respiratory Investigation 2016;54(6):428‐35. [2212‐5353] - PubMed
-
- Asai K, Minakata Y, Hirata K, Fukuchi Y, Kitawaki T, Ikeda K, et al. QVA149 once‐daily is safe and well tolerated and improves lung function and health status in Japanese patients with COPD: the ARISE study. European Respiratory Society 23rdAnnual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42:694s [P3392].
Berton 2009 {published data only}
-
- Berton D, Reis M, Siqueira A, Barroco A, Takara L, Bravo D. Comparative effects of formoterol monotherapy versus formoterol plus tiotropium on dynamic hyperinflation and exercise tolerance in COPD. European Respiratory Society 19th Annual Congress; 2009 Sep 12‐15; Vienna. 2009:[199].
-
- Berton DC, Chiappa GR, Takara LS, Figueiredo P, Reis M, Teixeira PJZ, et al. LABA‐LAMA combination is superior to LABA monotherapy in improving the cardiocirculatory responses to treadmill exercise in patients with moderate‐to‐severe COPD. European Respiratory Society 20th Annual Congress; 2010 Sep 18‐22; Barcelona. 2010:[E5411].
-
- Berton DC, Reis M, Siqueira AC, Barroco AC, Takara LS, Bravo DM, et al. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respiratory Medicine 2010;104(9):1288‐96. - PubMed
Buhl 2011 INTENSITY {published data only}
-
- Buhl R, Dunn LJ, Disdier C, Lassen C, Amos C, Henley M, et al. Blinded 12‐week comparison of once‐daily indacaterol and tiotropium in COPD. European Respiratory Journal 2011;38(4):797‐803. - PubMed
-
- Dunn L, Buhl R, Lassen C, Henley M, Kramer B. Blinded 12‐week comparison of once‐daily indacaterol and tiotropium in COPD. Chest 2010;138(4):719A. - PubMed
-
- NCT00900731. A study to compare the lung effect of indacaterol and tiotropium in chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT00900731 (first received 13 May 2009).
Buhl 2015 TONADO {published data only}
-
- Abrahams R, Ferguson G, Clerisme‐Beaty E, Groenke L, Voss F, Korducki L, et al. Effect of tiotropium + olodaterol on the use of nighttime rescue medication in patients with COPD: results from four randomized, double‐blind studies. Chest 2015;148(4):731A.
-
- Bai C, Buhl R, Lee SH, Lee KH, Jons O, Bothner U, et al. Safety of once‐daily tiotropium plus olodaterol via the respimat in East Asian patients with chronic obstructive pulmonary disease (COPD). Respirology 2015;20:55. [1323‐7799]
-
- Bai C, Buhl R, Lee SH, Lee KH, Jons O, Zhao Y, et al. Lung function with tiotropium plus olodaterol in East Asian patients with chronic obstructive pulmonary disease (COPD). Respirology 2015;20:53.
-
- Buhl R, Abrahams R, Bjermer L, Derom E, Flezar M, Hebert J, et al. Pooled safety analysis of once‐daily tiotropium and olodaterol fixed‐dose combination via the respimat in patients with chronic obstructive pulmonary disease: two 1‐year studies. Chest 2014;146(4):48A.
-
- Buhl R, Abrahams R, Bjermer L, Derom E, Flezar M, Hebert J, et al. Safety of once‐daily tiotropium and olodaterol fixed‐dose combination via the respimat in chronic obstructive pulmonary disease in two 1‐year studies. Thorax 2014;69:A189‐90.
D'Urzo 2014 AUGMENT {published data only}
-
- D'Urzo A, Mergel V, Leselbaum A, Caracta C. Efficacy and safety of fixed‐dose combination aclidinium bromide/formoterol fumarate in patients with COPD: results from the AUGMENT COPD trial. Chest 2013;144(4):1025A.
-
- D'Urzo A, Rennard S, Kerwin E, Mergel V, He T, Leselbaum A, et al. One‐year efficacy of aclidinium/formoterol fixed‐dose combination in COPD patients: the AUGMENT COPD study. European Respiratory Journal 2014;44:P286.
-
- D'Urzo A, Rennard S, Mergel V, Garcia Gil E, Leselbaum A, Caracta C. The augment COPD trial: efficacy and safety of a fixed‐dose combination of aclidinium bromide and formoterol fumarate in COPD patients. Chest 2014;145(3):426A.
-
- D'Urzo AD, Rennard SI, Kerwin EM, He T, Leselbaum A, Caracta C. Efficacy of fixed‐dose combination aclidinium bromide/formoterol fumarate on bronchodilation over 1 year: AUGMENT COPD extension trial in patients with moderate to severe COPD. American Journal of Respiratory and Critical Care Medicine 2014;189:A6006.
-
- D'Urzo AD, Rennard SI, Kerwin EM, Mergel V, Leselbaum A, Caracta C. Twice‐daily aclidinium bromide/formoterol fumarate fixed‐dose combination: lung function improvements in the AUGMENT COPD trial in patients with moderate to severe COPD. American Journal of Respiratory and Critical Care Medicine 2014;189:A3776.
Decramer 2014 {published data only}
-
- Anzueto A, Decramer M, Kaelin T, Richard N, Tabberer M, Harris S, et al. The efficacy and safety of umeclidinium/vilanterol compared with tiotropium or vilanterol over 24 weeks in subjects with COPD. American Journal of Respiratory and Critical Care Medicine 2013;187:A4268.
-
- Decramer M, Anzueto A, Kerwin E, Kaelin T, Richard N, Crater G, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respiratory Medicine 2014;2(6):472‐86. - PubMed
-
- Decramer M, Anzueto A, Kerwin E, Richard N, Crater G, Tabberer M, et al. Efficacy and safety of umeclidinium/vilanterol compared with umeclidinium or tiotropium in COPD. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42:751s [P3640].
-
- NCT01316900. 24‐week trial comparing GSK573719/GW642444 with GW642444 and with tiotropium in chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT01316900 (first received 16 March 2011).
-
- NCT01316913. 24‐week trial comparing GSK573719/GW642444 with GSK573719 and with tiotropium in chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT01316913 (first received 16 March 2011).
Di Marco 2005 {published data only}
-
- Marco F, Verga M, Santus P, Cazzola M, Mondoni M, Belloi E, et al. Formoterol (Modulite®), tiotropium and their combination in patients with acute exacerbations of chronic bronchitis: preliminary data. European Respiratory Journal 2005;26:1905.
-
- Marcoa F, Verga M, Santus P, Morelli N, Cazzola M, Centanni S. Effect of formoterol, tiotropium, and their combination in patients with acute exacerbation of chronic obstructive pulmonary disease: a pilot study. Respiratory Medicine 2006;11:1925‐32. - PubMed
Donohue 2013b {published data only}
-
- Donohue J, Niewoehner D, Brooks J, O'Dell D, Church A. Long‐term safety and tolerability of umeclidinium/vilanterol and umeclidinium in COPD. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42:144s [P760].
Donohue 2016 {published data only}
-
- Donohue J, Singh D, Munzu C, Kilbride S, Church A. Magnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: results from two randomised controlled trials. Respiratory Medicine 2016;112:65‐74. - PubMed
Donohue 2016b {published data only}
-
- Donohue JF, Soong W, Wu X, Shrestha P, Lei A. Long‐term safety of aclidinium bromide/formoterol fumarate fixed‐dose combination: results of a randomized 1‐year trial in patients with COPD. Respiratory Medicine 2016;116:41‐8. - PubMed
-
- Make B, Donohue J, Zhong X, Leselbaum A, Caracta C. Long‐term safety of a fixed‐dose combination of aclidinium bromide/formoterol fumarate in patients with stable moderate to severe COPD. Chest 2014;145(3):386A.
-
- Make B, Donohue JF, Soong W, Zhong X, Leselbaum A, Caracta C. Efficacy and tolerability of aclidinium bromide/formoterol fumarate fixed‐dose combination in patients with COPD: a 1‐year study. European Respiratory Journal 2014;44:P2413.
-
- Make BJ, Donohue JF, Soong W, Zhong X, Leselbaum A, Caracta C. Lung function and safety of aclidinium bromide/formoterol fumarate fixed‐dose combination: results of a 1‐year trial in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2014;189:A6010.
EUCTR2007‐003648‐31 {published data only}
-
- EUCTR2007‐003648‐31. A phase IIa, randomised, multicentre, evaluator‐blinded, 4‐way crossover clinical trial to study the pharmacokinetics, safety, tolerability and effects on lung function of one day treatment of formoterol 12 µg qd delivered by 2 different dry powder inhalers (Aerolizer® and Almirall Inhaler), of the fixed dose combination formoterol 12 µg + aclidinium bromide 200 µg qd delivered by Almirall Inhaler, and of formoterol 12 µg bid delivered by Aerolizer®, in moderate to severe chronic obstructive pulmonary disease patients. www.clinicaltrialsregister.eu/ctr‐search/trial/2007‐003648‐31/DE (first received 21 February 2008).
EUCTR2007‐004435‐30 {published data only}
-
- EUCTR2007‐004435‐30. A randomised, 4‐week, placebo‐controlled, double‐blind, 6 arm parallel group, dose‐finding clinical trial, to assess the efficacy and safety of three different doses of formoterol (6, 12 & 18 µg) combined with the inhaled anticholinergic aclidinium bromide 200 µg, aclidinium bromide 200 µg monotherapy and formoterol 12 µg monotherapy all administered once daily by inhalation via Almirall inhaler in patients with stable moderate to severe Chronic Obstructive Pulmonary Disease. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:200... (first received 14 April 2008).
EUCTR2009‐015901‐38 {published data only}
-
- EUCTR2009‐015901‐38. Efficacy, safety and tolerability of two fixed‐dose combinations of aclidinium bromide with two doses of formoterol fumarate compared with aclidinium bromide, formoterol fumarate and placebo all administered twice daily in stable, moderate to severe chronic obstructive pulmonary disease patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐015901‐38‐DE (first received 11 November 2009).
Evdokimov 2015 {published data only}
-
- Evdokimov V, Evdokimova A, Sheyanov M, Kozhina N. Clinical efficacy and safety of formoterol and tiotropium administration in patient with chronic heart failure due to coronary artery disease combined with chronic obstructive pulmonary disease. European Journal of Heart Failure 2015;17:68.
Evdokimov 2015b {published data only}
-
- Evdokimov V, Evdokimova A, Kovalenko E, Tebloev K. Prolonged use (18 months) of tiotropium and indacaterol in patient with chronic heart failure due to coronary artery disease combined with chronic obstructive pulmonary disease. European Journal of Heart Failure 2016;18:369. [1879‐0844]
-
- Evdokimov V, Evdokimova A, Kovalenko E, Tebloev K, Zolotova O. Clinical efficacy and safety of tiotropium and indacaterol administration in patient with chronic heart failure due to coronary artery disease combined with chronic obstructive pulmonary disease. European Heart Journal 2015;36:664.
-
- Evdokimov V, Evdokimova A, Tebloev K, Kovalenko E, Kozhina N, Zolotova O. Efficacy and safety of different types of bronchodilator therapy in patients with chronic heart failure due to coronary artery disease combined with chronic obstructive pulmonary disease. European Heart Journal 2014;35:385‐6.
-
- Evdokimov V, Evdokimova A, Tebloev K, Zolotova O. Clinical efficacy and safety of tiotropium and indacaterol administration in patient with chronic heart failure due to coronary artery disease combined with chronic obstructive pulmonary disease. European Journal of Heart Failure 2014;16:251. [1879‐0844]
Ferguson 2015 {published data only}
-
- Ferguson GT, Fowler‐Taylor A, Thach C, Wang Q, Schubert‐Tennigkeit AA, Banerji D. QVA149 is safe and well tolerated in patients with moderate‐to‐severe COPD: FLIGHT3, a 52 week safety study. American Journal of Respiratory and Critical Care Medicine 2015;191:A5762.
Fogarty 2014 {published data only}
-
- Fogarty C, Ortiz S. Pharmacokinetics, safety, and tolerability of aclidinium/formoterol fixed dose combination via pressair/genuair vs formoterol via foradil aerolizer in patients with moderate to severe COPD. Chest 2014;146(4 (2)):45A.
Hanania 2016 {published data only}
-
- Hanania NA, Tashkin DP, Kerwin E, Donohue J, Denenberg M, O'Donnell D, et al. Safety and efficacy of a novel LAMA/LABA co‐suspension technology glycopyrrolate/formoterol fixed‐dose combination delivered by MDI: results of a one‐year extension study in patients with COPD (PINNACLE‐3). American Journal of Respiratory and Critical Care Medicine 2016;193:A6791.
-
- Hanania NA, Tashkin DP, Kerwin EM, Donohue JF, Denenberg M, O'Donnell DE, et al. Long‐term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co‐Suspension Delivery Technology in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2017;126:105‐15. - PubMed
Hoshino 2014 {published data only}
-
- Hoshino M. Quantitative computed tomography assessment of airway dimensions by combining tiotropium and indacaterol in patients with COPD. European Respiratory Journal 2014;44:4668.
-
- Hoshino M, Ohtawa J. Computed tomography assessment of airway dimensions with combined tiotropium and indacaterol therapy in COPD patients. Respirology 2014;19(3):403‐10. - PubMed
Ichinose 2016 {published data only}
-
- Ichinose M, Minakata Y, Motegi T, Ueki J, Seki T, Anzai T, et al. Study design of VESUTO®: efficacy of tiotropium/olodaterol on lung hyperinflation, exercise capacity, and physical activity in Japanese patients with chronic obstructive pulmonary disease. Advances in Therapy 2017;34(7):1622‐35. - PMC - PubMed
-
- Ichinose M, Minakata Y, Motegi T, Ueki J, Seki T, Anzai T, et al. Study design of vesuto; study to evaluate the efficacy of tiotropium + olodaterol vs tiotropium on lung hyperinflation, exercise capacity, and physical activity in Japanese COPD patients. Respirology 2016;21:179.
-
- NCT02629965. Comparing the efficacy of tiotropium + olodaterol fixed dose combination (FDC) over tiotropium in improvement of lung hyperinflation, exercise capacity and physical activity in Japanese COPD patients. clinicaltrials.gov/ct2/show/NCT02629965 (first received 15 December 2015).
Ichinose 2017 {published data only}
-
- Ichinose M, Minakata Y, Motegi T, Ueki J, Seki T, Anzai T, et al. Study design of VESUTO: efficacy of tiotropium/olodaterol on lung hyperinflation, exercise capacity, and physical activity in Japanese patients with chronic obstructive pulmonary disease. Advance Therapeutics 2017;4(7):1622‐35. - PMC - PubMed
Imran 2015 {published data only}
-
- Imran M, Chhabra S, Kotwani A. Combinations of long acting beta<inf>2</inf> agonists to tiotropium: a randomized, double‐blind, placebo‐controlled, active‐drug controlled, parallel design academic clinical trial in moderate COPD male patients. Archives of Pharmacy Practice 2015;6(2):19‐23.
Jones 2010 {published data only}
-
- Jones CDS. The Combined Effect of Tiotropium and Formoterol on the Functional Status of Patients with Moderate‐to‐Severe COPD [Dissertation]. Charleston: Proquest, Umi Dissertation Publishing, 2010. [978‐112‐405‐5602602]
Mahler 2015 {published data only}
-
- Ayers T, Fogel R, Banerji D, Maitra S, Schubert‐Tennigkeit AA. QVA149, twice daily, is well tolerated in patients with moderate‐to‐severe COPD and has a safety profile similar to placebo: FLIGHT1 and FLIGHT2 pooled analysis in the subgroup of patients from the USA. Chest 2015;148(4):725A.
-
- Fogel R, Ayers T, Banerji D, Maitra S, Schubert‐Tennigkeit AA. Cardiovascular safety of QVA149 in patients with moderate‐to‐severe COPD: pooled analysis of FLIGHT1 and FLIGHT2 clinical studies. Chest 2015;148(4):718A.
-
- Fowler TA, Fogel R, Banerji D, Maitra S. Improvement in lung function, dyspnea, and health status with QVA149 compared with placebo in the subgroup of patients with COPD from the USA: FLIGHT1 and FLIGHT2 pooled analysis. Chest. F.‐Y. Chao, Emory University, Atlanta, GA, United States, 2015; Vol. 148, issue 4:718A.
-
- Jones P, Larbig M, Maitra S, Fowler‐Taylor A, Banerji D. Analysis of improvement in SGRQ component scores with QVA149: pooled data from the FLIGHT1 and FLIGHT2 studies. Chest 2015;148(4):722A.
-
- Jones PW, Ayers T, Fowler‐Taylor A, Larbig M, Thach C, Maitra S, et al. QVA149 demonstrates superior improvements in health status, as measured by SGRQ, compared with placebo in patients with moderate‐to‐severe COPD: the FLIGHT2 study. American Journal of Respiratory and Critical Care Medicine 2015;191:A5747.
Maltais 2010 {published data only}
-
- Maltais F, Beck E, Webster D, Maleki‐Yazdi MR, Seibt JV, Arnoux A, et al. Four weeks once daily treatment with tiotropium + olodaterol (BI 1744) fixed dose combination compared with tiotropium in COPD patients. European Respiratory Journal 2010;36(Suppl 54):1014S.
NCT00308191 2006 {published data only}
-
- NCT00308191. A phase IIIB study to compare the efficacy and safety of concomitant treatment in patients with COPD. clinicaltrials.gov/ct2/show/NCT00308191 (first received 29 March 2006).
NCT00424528 2006 {published data only}
-
- NCT00424528. Efficacy safety study of Arformoterol/Tiotropium combination versus either therapy alone in chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT00424528 (first received 19 January 2006).
NCT00696020 2008 {published data only}
-
- NCT00696020. Combination of orally inhaled BI1744CL/tiotropium bromide in patients with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT00696020 (first received 12 June 2008).
NCT00720499 2008 {published data only}
-
- NCT00720499. Efficacy and safety of 4 weeks of treatment with orally inhaled BI1744/tiotropium bromide in patients with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT00720499 (first received 22 July 2008).
NCT00845728 2009 {published data only}
-
- NCT00845728. Exacerbation study (INVIGORATE). clinicaltrials.gov/ct2/show/NCT00845728 (first received 18 February 2009).
NCT00846586 2009 {published data only}
-
- NCT00846586. Efficacy and safety of indacaterol plus tiotropium versus tiotropium alone in patients with chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT00846586 (first received 18 February 2009).
NCT00877383 2009 {published data only}
-
- NCT00877383. Efficacy and safety of indacaterol plus tiotropium versus tiotropium alone in patients with chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT00877383 (first received 7 April 2009).
NCT01040689 2010 {published data only}
-
- NCT01040689. Characterization of 24 hour spirometry profiles of inhaled BI 1744 CL and inhaled tiotropium bromide in patients with chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT01040689 (first received 30 December 2009).
NCT01040728 2010 {published data only}
-
- NCT01040728. A randomized, double‐blind, 4‐way crossover study to evaluate the efficacy of 24 hour spirometry profiles of inhaled BI 1744 CL and inhaled tiotropium bromide in patients with chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT01040728 (first received 30 December 2009).
NCT01049360 2009 {published data only}
-
- NCT01049360. Efficacy and safety study of two fixed‐dose combinations of aclidinium bromide with formoterol fumarate compared with aclidinium bromide, formoterol fumarate and placebo. clinicaltrials.gov/ct2/show/NCT01049360 (first received 14 January 2010).
NCT01437540 2011 {published data only}
-
- NCT01437540. Safety and tolerability of aclidinium bromide/formoterol fumarate compared with formoterol fumarate in patients with moderate to severe chronic obstructive pulmonary disease (LAC). clinicaltrials.gov/ct2/show/NCT01437540 (first received 21 September 2011).
NCT01476813 2012 {published data only}
-
- NCT01476813. Randomized cross over study to assess efficacy and safety of BDP/FF and glycopyrrolate. clinicaltrials.gov/ct2/show/NCT01476813 (first received 22 November 2011).
NCT01491802 2012 {published data only}
-
- NCT01491802. Effect of a new combination bronchodilator on exercise in GOLD stage II moderate COPD. clinicaltrials.gov/ct2/show/NCT01491802 (first received 14 December 2011).
NCT01529632 2012 {published data only}
-
- NCT01529632. Comparison of safety and efficacy of the combination product QVA149A against the concurrent administration of the individual components, QAB149 and NVA237, in patients with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT01529632 (first received 9 February 2012).
NCT01536262 2012 {published data only}
-
- NCT01536262. Japan long‐term safety for tiotropium plus olodaterol. clinicaltrials.gov/ct2/show/NCT01536262 (first received 22 February 2012).
NCT01551888 2012 {published data only}
-
- NCT01551888. Pharmacokinetic, safety and tolerability study of aclidinium/formoterol fixed dose combination and formoterol in patients with moderate to severe chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT01551888 (first received 13 March 2012).
NCT01574651 2012 {published data only}
-
- NCT01574651. The effect of QVA149 on health related quality of life in patients with chronic obstructive pulmonary disease (COPD) (QUANTIFY). clinicaltrials.gov/ct2/show/NCT01574651 (first received 10 April 2012).
NCT01682863 2012 {published data only}
-
- NCT01682863. A multi‐centre randomized double blind 52‐week study to assess the safety of QVA149 compared to QAB149 in patients with COPD who have moderate to severe airflow limitation. clinicaltrials.gov/ct2/show/NCT01682863 (first received 11 September 2012).
NCT01697696 2012 {published data only}
-
- NCT01697696. Long term safety study of NVA237 vs QAB149 in COPD patients. clinicaltrials.gov/ct2/show/NCT01697696 (first received 2 October 2012).
NCT01703845 2012 {published data only}
-
- NCT01703845. A study to characterize pharmacokinetics of tiotropium + olodaterol fixed‐dose combination in Japanese patients with COPD. clinicaltrials.gov/ct2/show/NCT01703845 (first received 11 October 2012).
NCT01817764 2013 {published data only}
-
- NCT01817764. A study to compare the efficacy and safety of umeclidinium/vilanterol and fluticasone propionate/salmeterol in subjects with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT01817764 (first received 25 March 2013).
NCT01985334 2014 {published data only}
-
- NCT01985334. Study to evaluate the efficacy and safety of glycopyrronium or indacaterol maleate and glycopyrronium bromide fixed‐dose combination regarding symptoms and health status in patients with moderate COPD switching from treatment with any standard COPD regimen. clinicaltrials.gov/ct2/show/NCT01985334 (first received 15 November 2013).
NCT02030535 2014 {published data only}
-
- NCT02030535. Study to evaluate the effect on lung function and ECG when a combination of tiotropium plus olodaterol is administered to patients with COPD either from a single inhaler or each compound is administered after each other from two different inhalers. clinicaltrials.gov/ct2/show/NCT02030535 (first received 8 January 2014).
NCT02059434 2013 {published data only}
-
- NCT02059434. Two‐part pharmacokinetic and pharmacodynamic study of LAS190792 in patients with asthma and COPD. clinicaltrials.gov/ct2/show/NCT02059434 (first received 11 February 2014).
NCT02196714 2014 {published data only}
-
- NCT02196714. PK study of PT003 and PT001 in Japanese healthy subjects. clinicaltrials.gov/ct2/show/NCT02196714 (first received 22 July 2014).
NCT02231177 2008 {published data only}
-
- NCT02231177. Pharmacokinetics and safety of BI 1744 CL plus tiotropium bromide in chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT02231177 (first received 4 September 2014).
NCT02296138 2015 {published data only}
-
- NCT02296138. Comparing the efficacy of tiotropium + olodaterol (5/5 µg) fixed dose combination (FDC) over tiotropium 5µg in reducing moderate to severe exacerbations in patients with severe to very severe chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT02296138 (first received 20 November 2014).
NCT02343458 2015 {published data only}
-
- NCT02343458. Study to assess the efficacy and safety of PT003, PT005, and PT001 in subjects with moderate to very severe COPD. clinicaltrials.gov/ct2/show/NCT02343458 (first received 22 January 2015).
NCT02429765 2015 {published data only}
-
- NCT02429765. Effect of aclidinium/formoterol on nighttime lung function and morning symptoms in chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT02429765 (first received 29 April 2015).
NCT02442206 2015 {published data only}
-
- NCT02442206. Effect of QVA149 (indacaterol maleate/glycopyrronium bromide) on cardiac function in COPD patients (CLAIM). clinicaltrials.gov/ct2/show/NCT02442206 (first received 13 May 2015).
NCT02465567 2015 {published data only}
-
- NCT02465567. Study to assess the efficacy and safety of PT010 relative to PT003 and PT009 in subjects with moderate to very severe COPD (Ethos). clinicaltrials.gov/ct2/show/NCT02465567 (first received 8 June 2015).
NCT02487446 2015 {published data only}
-
- NCT02487446. Efficacy and safety study of QVA149 in COPD patients. clinicaltrials.gov/ct2/show/NCT02487446 (first received 1 July 2015).
NCT02487498 2015 {published data only}
-
- NCT02487498. Efficacy and safety study of indacaterol maleate/glycopyrronium bromide in chronic obstructive pulmonary disease (COPD) patients. clinicaltrials.gov/ct2/show/NCT02487498 (first received 1 July 2015).
NCT02579850 2015 {published data only}
-
- NCT02579850. 2‐arm parallel group study of fixed combination of CHF 5993 vs ultibro® in COPD patients (TRIBUTE). clinicaltrials.gov/ct2/show/NCT02579850 (first received 20 October 2015).
NCT02643082 2015 {published data only}
-
- NCT02643082. A study to assess the effects of PT003 and placebo MDI on specific image based parameters in subjects with moderate to severe COPD. clinicaltrials.gov/ct2/show/NCT02643082 (first received 30 December 2015).
NCT02796677 2016 {published data only}
-
- NCT02796677. AMPLIFY ‐ D6571C00001 Duaklir USA phase III study. clinicaltrials.gov/ct2/show/NCT02796677 (first received 13 June 2016).
NCT02845752 2016 {published data only}
-
- NCT02845752. The effect of STIOLTO ™ RESPIMAT® on fatigue in chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT02845752 (first received 27 July 2016).
NCT02937584 2016 {published data only}
-
- NCT02937584. A study to assess the effects of PT001 and PT005 MDI on specific image based parameters in subjects with moderate to severe COPD. clinicaltrials.gov/ct2/show/NCT02937584 (first received 18 October 2016).
NCT02988869 2016 {published data only}
-
- NCT02988869. Tiotropium/formoterol Via Discair® vs tiotropium monotherapy or tiotropium + formoterol free combination treatment. clinicaltrials.gov/ct2/show/NCT02988869 (first received 9 December 2016).
NCT03022097 2017 {published data only}
-
- NCT03022097. Study to patients with stable COPD to assess the efficacy and safety of aclidinium bromide/formoterol fumarate (DUAKLIR) (AVANT). clinicaltrials.gov/ct2/show/NCT03022097 (first received 16 January 2017).
NCT03024346 2016 {published data only}
-
- NCT03024346. A study to assess the effects of PT003 and Placebo MDI on specific image based parameters in subjects with moderate to severe COPD. https://clinicaltrials.gov/ct2/show/NCT02643082 (note: NCT03024346 is an obsolete identifier referring to NCT02643082) (first received 30 December 2015).
NCT03034915 2017 {published data only}
-
- NCT03034915. A 24‐week study to compare umeclidinium/vilanterol (UMEC/VI), UMEC and salmeterol in subjects with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT03034915 (first received 27 January 2017).
Orevillo 2016 {published data only}
-
- Orevillo C, Miller J, DePetrillo P, Maes A, Siddiqui S, Reisner C. Pharmacokinetic and safety profile of the novel LAMA/LABA co‐suspension technology glycopyrrolate/formoterol fumarate fixed‐dose combination MDI, in Japanese healthy subjects. American Journal of Respiratory and Critical Care Medicine 2016;193:A6828.
Rabe 2015 {published data only}
-
- Martinez FJ, Rabe KF, Ferguson GT, Fabbri LM, Rennard S, Feldman GJ, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co‐suspension delivery technology in patients with COPD. Chest 2017;151(2):340‐57. - PubMed
-
- NCT01854658. Multi‐center study to assess the efficacy and safety of PT003, PT005, and PT001 in subjects with moderate to very severe COPD (PINNACLE 2). clinicaltrials.gov/ct2/show/NCT01854658 (first received 15 May 2013).
-
- Rabe K, Martinez F, Rodriguez‐Roisin R, Fabbri LM, Ferguson GT, Jones P, et al. PT003, a novel co‐suspension MDI glycopyrronium/formoterol fixed‐dose combination is superior to monocomponents in patients with COPD. European Respiratory Journal 2015;46:PA4363.
Reisner 2011 {published data only}
-
- Reisner C, Fogarty C, Spangenthal S, Dunn L, Kerwin E, Quinn D, et al. Novel combination of glycopyrrolate and formoterol MDI (GFF‐MDI) provides superior bronchodilation compared to its components administered alone, tiotropium DPI, and formoterol DPI in a randomized, double‐blind, placebo‐controlled phase 2b study In patients with COPD. American Journal of Respiratory and Critical Care Medicine 2011;183(1):A6435.
-
- Reisner C, Rennard SI, Fogarty C, Fischer T, Rose E, Fernandez C. Pearl therapeutics' combination LAMA/LABA MDI (GFF‐MDI, PT003) provides a significant benefit on home peak expiratory flow rate (PEFR) and reduces the need for rescue albuterol use compared to its components administered alone, Spiriva(R) Handihaler(R), and Foradil(R) Aerolizer(R) in a randomized, double‐blind, placebo‐controlled phase 2b study in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2012;185:A2259.
Reisner 2013 {published data only}
-
- Reisner C, Orevillo CJ, Fernandez C, Rose E, Kollar C, Rutty D, et al. Pearl Therapuetics' combination LAMA/LABA (GFF MDI, PT003) provides comparable safety as measured by 24‐hour holter monitoring to its components (GP MDI, PT001 And FF MDI, PT005) and Foradil ® Aerolizer®. American Journal of Respiratory and Critical Care Medicine 2013;187:A1485.
Reisner 2016 {published data only}
-
- Reisner C, Gottschlich G, Fakih F, Koser A, Krainson J, Delacruz L, et al. 24‐Hour lung function profile of novel co‐suspension technology glycopyrrolate/formoterol metered dose inhaler versus placebo and spiriva respimat, in patients with moderate‐to‐very‐severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2016;193:A6823.
Reisner 2017 {published data only}
-
- Reisner C, Fabbri L, Kerwin E, Fogarty C, Spangenthal S, Rabe K, et al. A randomized, seven‐day study to assess the efficacy and safety of a glycopyrrolate/formoterol fumarate fixed‐dose combination metered dose inhaler using novel Co‐SuspensionTM Delivery Technology in patients with moderate‐to‐very severe chronic obstructive pulmonary disease. Respiratory Research 2017;18(1):8. [1465‐993X] - PMC - PubMed
-
- Reisner C, Fabbri LM, Kerwin EM, Fogarty C, Spangenthal S, Rabe KF, et al. A randomized, seven‐day study to assess the efficacy and safety of a glycopyrrolate/formoterol fumarate fixed‐dose combination metered dose inhaler using novel Co‐Suspension™ Delivery Technology in patients with moderate‐to‐very severe chronic obstructive pulmonary disease. Respiratory Research 2017;18(1):8. - PMC - PubMed
-
- Reisner C, Fernandez C, Orevillo C, Rose E, Kollar C. Pearl Therapeutics' combination LAMA/LABA MDI (GFF MDI, PT003) provides comparable metabolic and ECG safety to Spiriva(R) handihaler(R) and placebo In patients with COPD. American Journal of Respiratory and Critical Care Medicine 2012;185:A2925.
Reisner 2017b {published data only}
-
- NCT02347072. 24‐hour lung function in subjects with moderate to very severe COPD after treatment with PT003, open‐label Spiriva® Respimat® as an active control, and placebo. clinicaltrials.gov/ct2/show/NCT02347072 (first received 27 January 2015).
-
- NCT02347085. 24‐hour lung function in subjects with moderate to very severe COPD after treatment with PT003 and placebo MDI. clinicaltrials.gov/ct2/show/NCT02347085 (first received 27 January 2015).
-
- Reisner C, Gottschlich G, Fakih F, Koser A, Krainson J, Delacruz L, et al. 24‐h bronchodilation and inspiratory capacity improvements with glycopyrrolate/formoterol fumarate via co‐suspension delivery technology in COPD. Respiratory Research 2017;18(1):157. [DOI: 10.1186/s12931-017-0636-4] - DOI - PMC - PubMed
Sadigov 2014 {published data only}
-
- Sadigov A, Akhundov S, Bagirov R. Analysis of chronic obstructive pulmonary disease exacerbations with the triple therapy compared with dual and single bronchodilator therapy: which treatment is better for patients with severe disease?. Chest 2014;145(3):425A.
Salomon 2017 {published data only}
-
- NCT01699685. Swiss studY for the treatment of COPD patients with the free combiNation of indacatERol and GlYcopyrroniumbromide (SYNERGY). clinicaltrials.gov/ct2/show/NCT01699685 (first received 4 October 2012).
Setoguchi 2015 {published data only}
-
- Setoguchi Y. Indactaterol mono‐and combination therapy with inhaled tiotropium bromide in COPD: a 52‐week study. American Journal of Respiratory and Critical Care Medicine 2015;191:A5748.
-
- Setoguchi Y. The combination therapy with inhaled tiotropium bromide and indacaterol in COPD improves the peripheral airway function in static and dynamic status. European Respiratory Journal 2014;44:P896.
Singh 2014 {published data only}
-
- Bateman ED, Chapman KR, Singh D, D'Urzo AD, Molins E, Leselbaum A, et al. Aclidinium bromide and formoterol fumarate as a fixed‐dose combination in COPD: pooled analysis of symptoms and exacerbations from two six‐month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respiratory Research 2015;16:92. [1465‐993X] - PMC - PubMed
-
- Jones PW, Singh D, Bateman ED, Korn S, Serra C, Molins E, et al. The effect of aclidinium/formoterol fixed‐dose combination on COPD symptoms and health status in patients with COPD: results from the ACLIFORM/COPD study. American Journal of Respiratory and Critical Care Medicine 2014;189:A3764.
-
- Miravitlles M, Chapman KR, Chuecos F, Ribera A, Garcia Gil E. The efficacy of aclidinium/formoterol on lung function and symptoms in patients with COPD categorized by symptom status: a pooled analysis. International Journal of Chronic Obstructive Pulmonary Disease 2016;11(1):2041‐52. [1178‐2005] - PMC - PubMed
-
- Miravitlles M, Garcia Gil E, Chuecos F, Lamarca R. Morning and nighttime symptoms in COPD and efficacy of aclidinium/formoterol in symptomatic vs asymptomatic patients. European Respiratory Journal 2015;46:OA1968.
-
- NCT01462942. Long‐term efficacy and safety of aclidinium bromide/formoterol fumarate fixed‐dose combination. clinicaltrials.gov/ct2/show/NCT01462942 (first received 1 November 2011).
Sliwinski 2010 {published data only}
-
- Sliwinski P, Perng DW, Chuchalin A, Jones PW. Efficacy and safety of once‐daily aclidinium bromide 200 muG in combination with formoterol in patients with COPD. Thorax 2010;65:A136 [P137].
Tanaka 2015 {published data only}
-
- Tanaka Y, Hino M, Gemma A, Kobayashi Y, Katoh Y. 2nd interim report: Effect of tiotropium monotherapy versus tiotropium plus indacaterol in patients with chronic obstructive pulmonary disease ‐ single‐centre, randomized, prospective, real‐world study. Respirology 2015;20:37. [1323‐7799]
-
- Tanaka Y, Hino M, Naitoh T, Aoyama J, Kato Y, Kosaihira S, et al. 3rd interim report: Effect of tiotropium monotherapy versus tiotropium plus indacaterol in patients with chronic obstructive pulmonary disease ‐ single‐centre, randomised, prospective, real‐world study. Respirology 2016;21:176. [1323‐7799]
Tashkin 2007 {published data only}
-
- NCT00139932. Tiotropium bromide alone vs tiotropium bromide and formoterol fumarate in subjects with COPD (study P04272). clinicaltrials.gov/ct2/show/NCT00139932 (first received 31 August 2005).
-
- Tashkin D. Formoterol and tiotropium reduces rescue medication use more than tiotropium alone in patients with moderate COPD: findings from a 12 week randomized placebo controlled trial. American Thoracic Society International Conference; 2008 May 16‐21; Toronto. 2008:A647[#F5].
-
- Tashkin D, Pearle J, Iezzoni D, Varghese S. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD 2009;6(1):17‐25. [1541‐2563] - PubMed
-
- Tashkin D, Pearle J, Varghese S. Improvement of lung function with coadministered formoterol and tiotropium, regardless of smoking status in patients with chronic obstructive pulmonary disease. Chest 2008;134(4):103002s.
-
- Tashkin D, Varghese S. Combined treatment with formoterol and tiotropium is more efficacious than treatment with tiotropium alone in patients with chronic obstructive pulmonary disease, regardless of smoking status, inhaled corticosteroid use, baseline severity, or gender. Pulmonary Pharmacology and Therapeutics 2011;24(1):147‐52. [1094‐5539] - PubMed
Tashkin 2016 {published data only}
-
- Reisner C, Gotfried M, Denenberg MB, Fernandez C, Rose E, Fortner P, et al. Low doses of Pearl Therapeutics' LAMA/LABA Combination MDI (GFF MDI, PT003) provide superior bronchodilation compared to components and to open‐label spiriva handihaler in a randomized, double‐blind, placebo‐controlled phase IIb study in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2013;187:A2434.
-
- Tashkin DP, Martinez FJ, Rodriguez‐Roisin R, Fogarty C, Gotfried M, Denenberg M, et al. A multicenter, randomized, double‐blind dose‐ranging study of glycopyrrolate/formoterol fumarate fixed‐dose combination metered dose inhaler compared to the monocomponents and open‐label tiotropium dry powder inhaler in patients with moderate‐to‐severe COPD. Respiratory Medicine 2016;120:16‐24. - PubMed
Ulubay 2005 {published data only}
-
- Ulubay G, Eyuboglu Oner F, Savas Bozbas S, Simsek A. Three regimens of inhaled bronchodilators for chronic obstructive pulmonary disease: comparison of pulmonary function and cardiopulmonary exercise test parameters. Turkish Respiratory Journal 2005;6(2):89‐94.
Van de Maele 2010 {published data only}
-
- Fabbri LM, Maele B, Lemmens B, Martin C, Horton R, Dolker M, et al. Cardiovascular safety of qva149, a novel combination of indacaterol and glycopyrronium, compared with indacaterol and placebo in patients with COPD. European Respiratory Society 19th Annual Congress; 2009 Sep 12‐15; Vienna. 2009:[P2027].
-
- Maele B, Fabbri LM, Martin C, Horton R, Dolker M, Overend T. Cardiovascular safety of QVA149, a combination of Indacaterol and NVA237, in COPD patients. COPD 2010;7:418‐27. - PubMed
Van Noord 2005 {published data only}
-
- Noord J, Aumann J, Janssens E, Mueller A, Cornelissen P. Relation between the acute response to salbutamol and long term FEV1 responses to tiotropium (TIO), formoterol (FORM) and its combination (T=F) in COPD patients. European Respiratory Journal 2003;22:153.
-
- Noord J, Qumann J, Janssens E, Mueller A, Cornelissen P, Aumann J, et al. Comparison of once daily tiotropium, twice daily formoterol and the free combination, once daily, in patients with COPD. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle. 2003:B024 Poster 421.
-
- Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. European Respiratory Journal 2005;26(2):214‐22. - PubMed
Van Noord 2010 {published data only}
-
- Noord J, Buhl R, Laforce C, Martin C, Jones F, Dolker M, et al. QVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary disease. Thorax 2010;65(12):1086‐91. - PubMed
-
- Noord JA, Buhl R, LaForce C, Martin C, Jones F, Dolker M, et al. Qva149, a novel combination of indacaterol and glycopyrronium, demonstrates superior bronchodilation compared with indacaterol or placebo in patients with COPD. European Respiratory Society 19th Annual Congress; 2009 Sep 12‐15; Vienna. 2009:[E4347].
Velazquez‐Uncal 2016 {published data only}
-
- Velazquez‐Uncal M, Ramirez‐Venegas A, Sansores R, Perez‐Bautista O, Hernandez‐Zenteno R, Velazquez A, et al. Clinically significant impact of tiotropium and indacaterol on six‐minute walking test and inspiratory capacity in women with chronic obstructive pulmonary disease associated to biomass exposure. American Journal of Respiratory and Critical Care Medicine 2016;193:A3534.
Vincken 2013 {published data only}
-
- NCT01604278. Efficacy, safety and tolerability of the co‐administration of NVA237 plus indacaterol once daily versus indacaterol once daily in patients with moderate to severe chronic obstructive pulmonary disease (COPD) (GLOW6). clinicaltrials.gov/ct2/show/NCT01604278 (first received 23 May 2012).
-
- Vincken W, Aumann J, Jack D, Chen H, Henley M, Goyal P. Once‐daily co‐administration of glycopyrronium and indacaterol via Breezhaler® device improves lung function and symptoms in patients with COPD versus indacaterol alone: the GLOW6 study. Thorax. 2013:A181 [P232].
Vogelmeier 2008 {published data only}
-
- Arievich H, Potena A, Fonay K, Vogelmeier CF, Overend T, Smith J, et al. Formoterol given either alone or together with tiotropium reduces the rate of exacerbations in stable COPD patients. European Respiratory Journal 2006;28:440s [P2514].
-
- Vogelmeier C, Kardos P, Harari S, Gans S, Stenglein S, Thirlwell J. Formoterol mono‐ and combination therapy with tiotropium in patients with COPD: a 6‐month study. Respiratory Medicine 2008;102(11):1511‐20. - PubMed
-
- Vogelmeier CF, Harari SA, Fonay K, Beier J, Overend T, Till D, et al. Formoterol and tiotropium both improve lung function in stable COPD patients with some additional benefit when given together. European Respiratory Journal 2006;28:429s [P2506].
Vogelmeier 2013 {published data only}
-
- Vogelmeier C, Bateman E, Pallante J, Bryant H, Alagappan V, D'Andrea P, et al. QVA149 once daily is safe and well tolerated in patients with COPD: the ILLUMINATE study. American Journal of Respiratory and Critical Care Medicine 2013;187:A1477.
-
- Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D'Andrea P, Chen H, et al. Efficacy and safety of once‐daily QVA149 compared with twice‐daily salmeterol‐fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double‐blind, parallel group study. Lancet Respiratory Medicine 2013;1(1):51‐60. - PubMed
Watz 2017 {published data only}
-
- Aymerich JG, Watz H, Beeh KM, Paggiaro P, Moya M, Notari M, et al. Use of the Daily PROactive instrument to evaluate physical activity in patients with COPD: results from ACTIVATE. European Respiratory Journal 2017;50:PA4964.
-
- NCT02424344. Effect of aclidinium/formoterol on lung hyperinflation, exercise capacity and physical activity in moderate to severe COPD patients. clinicaltrials.gov/ct2/show/NCT02424344 (first received 23 April 2015).
-
- Watz H, Troosters T, Beeh KM, Garcia‐Aymerich J, Paggiaro P, Molins E, et al. ACTIVATE: the effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:2545‐58. - PMC - PubMed
Webb 2015 {published data only}
-
- Webb KA, Ciavaglia CE, Preston M, O'Donnell DE. Effects of dual umeclidinium/vilanterol compared to umeclidinum on exertional dyspnea, respiratory mechanics and neural drive in patients with moderate COPD. American Journal of Respiratory and Critical Care Medicine 2015;191:A5735.
Wedzicha 2013 {published data only}
-
- CQVA149A2303. A 26‐week treatment, multi‐center, randomized, double‐blind, parallel‐group, placebo and active controlled (open label) study to assess the efficacy, safety and tolerability of QVA149 (110/50 μg q.d.) in patients with moderate to severe chronic obstructive pulmonary disease (COPD). www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=7983 (first received 21 September 2010).
-
- Decramer M, Wedzicha JA, Ficker J, Fowler‐Taylor A, D'Andrea P, Arrasate C, et al. Once‐daily QVA149 improves health‐related quality of life in patients with severe to very severe COPD: the Spark study. American Journal of Respiratory and Critical Care Medicine 2013;187:A4257.
-
- Ficker JH, Wedzicha JA, Decramer M, Fowler‐Taylor A, D'Andrea P, Arrasate C, et al. QVA149 reduces COPD exacerbation rate in various subgroups of patients: the SPARK study. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42:3s [182].
-
- Mezzi K, Wedzicha J, Decramer M, Fowler‐Taylor A, Fogel R, Chen H, et al. QVA149 provides greater improvement in patient reported symptom scores compared to glycopyrronium and tiotropium in patients with severe to very severe COPD: the SPARK study [Abstract]. 7th International Primary Care Respiratory Group (IPCRG) World Conference; 2014 May 21‐24; Athens. 2014:PO005‐FRI.
-
- Meerhaeghe A, Vanhaeverbeek M. QVA149 versus glycopyrronium for COPD. Lancet Respiratory Medicine 2013;1(5):e22‐3. [2213‐2600] - PubMed
Yosuke 2014 {published data only}
-
- Yosuke T, Mitsunori H, Naomi O, Hiroyuki T, Seiji K, Norihisa M, et al. Comparison of monotherapy with a long‐acting muscarinic antagonist versus combination therapy with a long‐acting muscarinic antagonist plus a long‐acting beta2‐agonist in patients with chronic obstructive pulmonary disease. Respirology 2014;19:122.
ZuWallack 2014 {published data only}
-
- NCT01694771. Co‐administration of olodaterol Respimat® and tiotropium Handihaler®. clinicaltrials.gov/ct2/show/NCT01694771 (first received 4 September 2014).
-
- NCT01696058. Co‐administration of olodaterol Respimat® and tiotropium Handihaler®. clinicaltrials.gov/ct2/show/NCT01696058 (first received 28 September 2012).
-
- Wallack RZ, Allen L, Hernandez G, Ting N, Abrahams R. Health status of patients with COPD receiving the combination of olodaterol respimat and tiotropium handihaler compared with tiotropium: results from two replicate, randomized, double‐blind studies. Chest 2014;146(4):46A.
-
- ZuWallack R, Allen L, Hernandez G, Ting N, Abrahams R. Efficacy and safety of combining olodaterol Respimat((R)) and tiotropium HandiHaler((R)) in patients with COPD: results of two randomized, double‐blind, active‐controlled studies. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:1133‐44. - PMC - PubMed
References to studies awaiting assessment
NCT02233543 2014 {published data only}
-
- NCT02233543. A multicenter, 4‐week crossover (total duration 12 weeks), placebo‐controlled, double‐blind study to determine the impact of QVA149 (indacaterol/glycopyrronium) 85/43 µg on nocturnal oxygen levels in chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT02233543 (first received 8 September 2014).
Additional references
Aaron 2007
-
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone‐salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2007;146(8):545‐55. - PubMed
Aaron 2008
-
- Aaron SD, Fergusson D, Marks GB, Suissa S, Vandemheen KL, Doucette S, et al. Counting, analysing and reporting exacerbations of COPD in randomised controlled trials. Thorax 2008;63(2):122‐8. - PubMed
Bangalore 2007
-
- Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed‐dose combinations improve medication compliance: a meta‐analysis. American Journal of Medicine 2007;120(8):713‐9. - PubMed
Barnes 2015
-
- Barnes PJ, Burney PGJ, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, et al. Chronic obstructive pulmonary disease. Nature Reviews Disease Primers 2015;122:15076. - PubMed
Bateman 2015
-
- Bateman ED, Chapman KR, Singh D, D’Urzo AD, Molins E, Leselbaum A, et al. Aclidinium bromide and formoterol fumarate as a fixed‐dose combination in COPD: pooled analysis of symptoms and exacerbations from two six‐month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respiratory Research 2015;16(1):92. - PMC - PubMed
Beeh 2016
-
- Beeh KM, Burgel P‐R, Franssen FME, Lopez‐Campos JL, Loukides S, Hurst JR, et al. How do dual long‐acting bronchodilators prevent exacerbations of chronic obstructive pulmonary disease?. American Journal of Respiratory and Critical Care Medicine 2016;196(2):139‐49. - PubMed
Calverley 2005
-
- Calverley PMA. Minimal clinically important difference ‐ exacerbations of COPD. COPD 2005;2(1):143‐8. - PubMed
Calzetta 2015
-
- Calzetta L, Matera MG, Cazzola M. Pharmacological interaction between LABAs and LAMAs in the airways: optimizing synergy. European Journal of Pharmacology 2015;761(C):168–73. - PubMed
Calzetta 2016
-
- Calzetta L, Rogliani P, Matera MG, Cazzola M. A systematic review with meta‐analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest 2016;149(5):1181‐96. - PubMed
Calzetta 2017
Cazzola 2008
-
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. European Respiratory Journal 2008;31(2):416‐69. - PubMed
Cazzola 2010
-
- Cazzola M, Molimard M. The scientific rationale for combining long‐acting & beta2‐agonists and muscarinic antagonists in COPD. Pulmonary Pharmacology 2010;23(4):257–67. - PubMed
Cazzola 2015
-
- Cazzola M, Calzetta L, Ora J, Puxeddu E, Rogliani P, Matera MG. Searching for the synergistic effect between aclidinium and formoterol: from bench to bedside.. Respiratory Medicine 2015;109(10):1305‐11. - PubMed
CDC 2016
-
- Center for Disease Control. Chronic obstructive pulmonary disease among adults ‐ United States, 2016. www.cdc.gov/copd/index.html (accessed 1 Apr 2017).
Chapman 2013
-
- Chapman KR, Bergeron C, Bhutani M, Bourbeau J, Grossman RF, Hernandez P, et al. Do we know the minimal clinically important difference (MCID) for COPD exacerbations?. COPD 2013;10(2):243‐9. - PubMed
Donohue 2005
-
- Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005;2(1):111‐24. - PubMed
Farne 2015
GOLD 2017
-
- Global Initiative for Chronic Obstructive Lung Disease. From the global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease (GOLD) 2018. goldcopd.org/gold‐reports/ (accessed prior to 28 September 2018).
Guyatt 1987
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Jones 1992
-
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self‐complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. American Review of Respiratory Disease 1992;145(6):1321‐7. - PubMed
MECIR 2018
-
- Higgins J, Lasserson T, Chandler J, Tovey D, Churchill R. Standards for the conduct and reporting of new Cochrane Intervention Reviews, reporting of protocols and the planning, conduct and reporting of updates (Version 1.05). community.cochrane.org/mecir‐manual (accessed prior to 28 September 2018).
Oba 2016
-
- Oba Y, Sarva ST, Dias S. Efficacy and safety of long‐acting β‐agonist/long‐acting muscarinic antagonist combinations in COPD: a network meta‐analysis. Thorax 2016;71(1):15‐25. - PubMed
Polkey 2013
-
- Polkey MI, Spruit M, Edwards LD, Watkins ML, Pinto‐Plata V, Vestbo JÃ, et al. Six‐minute‐walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. American Journal of Respiratory and Critical Care Medicine 2013;187(4):382‐6. - PubMed
Puhan 2006
Quaseem 2011
-
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155:179‐91. - PubMed
Sarai 2014
-
- Sarai M, Sin D, FitzGerald JM, Aaron S. Long‐acting beta2‐agonists and long‐acting muscarinic antagonists in a combined inhaler versus either agent alone or placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011282] - DOI - PMC - PubMed
Schunemann 2003
-
- Schunemann HJ, Griffith L, Jaeschke R, Goldstein R, Stubbing D, Guyatt GH. Evaluation of the minimal important difference for the feeling thermometer and the St. George's Respiratory Questionnaire in patients with chronic airflow obstruction. Journal of Clinical Epidemiology 2003;56(12):1170‐6. - PubMed
Schunemann 2005
-
- Schunemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the chronic respiratory disease questionnaire (CRQ). COPD 2005;2(1):81‐9. - PubMed
SGRQ‐C Manual
-
- Jones P. St George’s Respiratory Questionnaire for COPD patients (SGRQ‐C). www.healthstatus.sgul.ac.uk/SGRQ_download/SGRQ‐C%20Manual%20March%202016... (accessed 10 May 2018).
Singh 2014a
-
- Singh D, Jones PW, Bateman ED, Korn S, Serra C, Molins E, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed‐dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM‐COPD): a multicentre, randomised study. BMC Pulmonary Medicine 2014;14:178. - PMC - PubMed
Singh 2014b
-
- Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. European Respiratory Journal 2014;44:1447‐78. - PubMed
Vestbo 2013
-
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2013;187(4):347–65. - PubMed
Wedzicha 2014
-
- Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandström T, Taylor AF, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double‐blind, parallel‐group study. Lancet Respiratory Medicine 2013;1(3):199–209. - PubMed
Wedzicha 2017
-
- Wedzicha JA, Calverley PMA, Albert RK, Anzueto A, Criner GJ, Hurst JR, et al. Prevention of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. European Respiratory Journal 2017;50(3):1602265. - PubMed
Westwood 2011
WHO 2015
-
- World Health Organization. Global health estimates ‐ year 2015. www.who.int/mediacentre/factsheets/fs310/en/ (accessed 1 April 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

