Crystal structure of the legume lectin-like domain of an ERGIC-53-like protein from Entamoeba histolytica
- PMID: 30839295
- PMCID: PMC6404861
- DOI: 10.1107/S2053230X19000499
Crystal structure of the legume lectin-like domain of an ERGIC-53-like protein from Entamoeba histolytica
Abstract
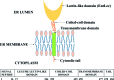
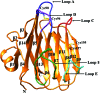
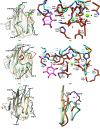
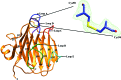
ERGIC-53-like proteins are type I membrane proteins that belong to the class of intracellular cargo receptors and are known to be indispensable for the intracellular transport of glycoproteins. They are implicated in transporting glycoproteins between the endoplasmic reticulum and the Golgi body. The crystal structure of the legume lectin-like domain of an ERGIC-53-like protein from Entamoeba histolytica has been determined at 2.4 Å resolution. Although the overall structure of the domain resembles those of its mammalian and yeast orthologs (ERGIC-53 and Emp46, respectively), there are significant changes in the carbohydrate-binding site. A sequence-based search revealed the presence of several homologs of ERGIC-53 in different species of Entamoeba. This is the first report of the structural characterization of a member of this class of proteins from a protozoan and serves to further knowledge and understanding regarding the species-specific differences.
Keywords: ERGIC-53-like proteins; Entamoeba histolytica; intracellular cargo receptors; legume lectin-like domains; membrane proteins.
Figures

Similar articles
-
Crystal structures of a β-trefoil lectin from Entamoeba histolytica in monomeric and a novel disulfide bond-mediated dimeric forms.Glycobiology. 2020 Jul 20;30(7):474-488. doi: 10.1093/glycob/cwaa001. Glycobiology. 2020. PMID: 31967310
-
Expression, purification, refolding and crystallization of the carbohydrate-recognition domain of p58/ERGIC-53, an animal C-type lectin involved in export of glycoproteins from the endoplasmic reticulum.Acta Crystallogr D Biol Crystallogr. 2002 Mar;58(Pt 3):536-8. doi: 10.1107/s0907444902000203. Epub 2002 Feb 21. Acta Crystallogr D Biol Crystallogr. 2002. PMID: 11856848
-
Structures of the carbohydrate recognition domain of Ca2+-independent cargo receptors Emp46p and Emp47p.J Biol Chem. 2006 Apr 14;281(15):10410-9. doi: 10.1074/jbc.M512258200. Epub 2006 Jan 26. J Biol Chem. 2006. PMID: 16439369
-
Structure and function of the Entamoeba histolytica Gal/GalNAc lectin.Int Rev Cytol. 2002;216:59-80. doi: 10.1016/s0074-7696(02)16003-7. Int Rev Cytol. 2002. PMID: 12049210 Review.
-
Lectins and protein traffic early in the secretory pathway.Biochem Soc Symp. 2002;(69):73-82. doi: 10.1042/bss0690073. Biochem Soc Symp. 2002. PMID: 12655775 Review.
Cited by
-
A Review: Natural and Synthetic Compounds Targeting Entamoeba histolytica and Its Biological Membrane.Membranes (Basel). 2022 Apr 1;12(4):396. doi: 10.3390/membranes12040396. Membranes (Basel). 2022. PMID: 35448367 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

