Neuromuscular dysfunction, independent of gait dysfunction, modulates trabecular bone homeostasis in mice
- PMID: 30839306
- PMCID: PMC6454260
Neuromuscular dysfunction, independent of gait dysfunction, modulates trabecular bone homeostasis in mice
Abstract
Objectives: To clarify the effects of neuromuscular dysfunction on hindlimb loading, muscle atrophy, and bone homeostasis.
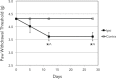
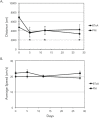
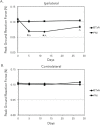
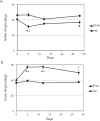
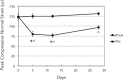
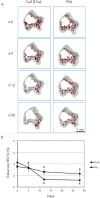
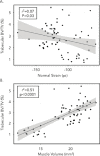
Methods: We quantified changes to hindlimb loading, muscle atrophy, and bone morphology following either Botulinum toxin A (BTxA) induced muscle paralysis or peripheral nerve injury (PNI) in mice; two in vivo models that we anticipated would differently alter gait and mechanical loading patterns due to their distinct effects on neuromuscular signaling. To confirm the expected behavioral effects of PNI, we assessed mechanical allodynia of the ipsilateral hindlimb using von Frey testing and activity (distance traveled and speed) was monitored in both groups using open field testing. Peak vertical ground reaction forces (GRF) and ankle and knee kinematics during normal locomotion were quantified and used to estimate peak mid-diaphyseal normal strains. Muscle atrophy and trabecular and cortical bone morphology were assessed via high-resolution microCT imaging.
Results: BTxA-induced calf paralysis caused severe muscle atrophy and altered gait kinetics and kinematics and reduced gait-induced normal strains. PNI increased mechanical allodynia but did not alter gait, nor did it cause muscle atrophy. We observed that muscle paralysis and PNI both led to severe trabecular bone loss but only BTxA-induced paralysis increased cortical bone resorption.
Conclusions: While mechanical stimuli clearly have essential functions in bone development and adaptation, these data emphasize that neuromuscular signaling, independent of load-induced mechanical strains, may modulate trabecular bone homeostasis in normal and disease states.
Conflict of interest statement
The authors have no conflict of interest.
Figures


Similar articles
-
Botulinum Toxin-induced Muscle Paralysis Inhibits Heterotopic Bone Formation.Clin Orthop Relat Res. 2015 Sep;473(9):2825-30. doi: 10.1007/s11999-015-4271-4. Clin Orthop Relat Res. 2015. PMID: 25804882 Free PMC article.
-
Transient muscle paralysis disrupts bone homeostasis by rapid degradation of bone morphology.Bone. 2010 Jan;46(1):18-23. doi: 10.1016/j.bone.2009.10.025. Epub 2009 Oct 24. Bone. 2010. PMID: 19857614 Free PMC article.
-
Botox induced muscle paralysis rapidly degrades bone.Bone. 2006 Feb;38(2):257-64. doi: 10.1016/j.bone.2005.08.009. Epub 2005 Sep 26. Bone. 2006. PMID: 16185943 Free PMC article.
-
[Botulinum toxin A and musculoskeletal pain].Ann Readapt Med Phys. 2003 Jul;46(6):329-32. doi: 10.1016/s0168-6054(03)00109-0. Ann Readapt Med Phys. 2003. PMID: 12928139 Review. French.
-
Mandibular Bone Loss after Masticatory Muscles Intervention with Botulinum Toxin: An Approach from Basic Research to Clinical Findings.Toxins (Basel). 2019 Feb 1;11(2):84. doi: 10.3390/toxins11020084. Toxins (Basel). 2019. PMID: 30717172 Free PMC article. Review.
Cited by
-
Dominant and nondominant distal radius microstructure: Predictors of asymmetry and effects of a unilateral mechanical loading intervention.Bone Rep. 2021 Mar 13;14:101012. doi: 10.1016/j.bonr.2021.101012. eCollection 2021 Jun. Bone Rep. 2021. PMID: 33786342 Free PMC article.
-
The Efficacy of PTH and Abaloparatide to Counteract Immobilization-Induced Osteopenia Is in General Similar.Front Endocrinol (Lausanne). 2020 Oct 9;11:588773. doi: 10.3389/fendo.2020.588773. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33162940 Free PMC article.
-
Beta 2 Adrenergic Receptor Selective Antagonist Enhances Mechanically Stimulated Bone Anabolism in Aged Mice.JBMR Plus. 2022 Dec 27;7(2):e10712. doi: 10.1002/jbm4.10712. eCollection 2023 Feb. JBMR Plus. 2022. PMID: 36751418 Free PMC article.
-
Botulinum Toxin A and Osteosarcopenia in Experimental Animals: A Scoping Review.Toxins (Basel). 2021 Mar 14;13(3):213. doi: 10.3390/toxins13030213. Toxins (Basel). 2021. PMID: 33799488 Free PMC article.
-
Strategies for Improving Impaired Osseointegration in Compromised Animal Models.J Dent Res. 2024 May;103(5):467-476. doi: 10.1177/00220345241231777. Epub 2024 Apr 15. J Dent Res. 2024. PMID: 38616679 Free PMC article. Review.
References
-
- Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12(10):1547–51. - PubMed
-
- Gross TS, Poliachik SL, Prasad J, Bain SD. The effect of muscle dysfunction on bone mass and morphology. J Musculoskelet Neuronal Interact 2010; in press - PubMed
-
- Frost HM. Bone “mass” and the “mechanostat”:a proposal. Anat Rec. 1987;219(1):1–9. - PubMed
-
- Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates;an alternative to allometric limb bone scaling. J Theor Biol. 1984;107(2):321–7. - PubMed
