α2δ-1-Bound N-Methyl-D-aspartate Receptors Mediate Morphine-induced Hyperalgesia and Analgesic Tolerance by Potentiating Glutamatergic Input in Rodents
- PMID: 30839350
- PMCID: PMC6469992
- DOI: 10.1097/ALN.0000000000002648
α2δ-1-Bound N-Methyl-D-aspartate Receptors Mediate Morphine-induced Hyperalgesia and Analgesic Tolerance by Potentiating Glutamatergic Input in Rodents
Abstract
Background: Chronic use of μ-opioid receptor agonists paradoxically causes both hyperalgesia and the loss of analgesic efficacy. Opioid treatment increases presynaptic N-methyl-D-aspartate receptor activity to potentiate nociceptive input to spinal dorsal horn neurons. However, the mechanism responsible for this opioid-induced activation of presynaptic N-methyl-D-aspartate receptors remains unclear. α2δ-1, formerly known as a calcium channel subunit, interacts with N-methyl-D-aspartate receptors and is primarily expressed at presynaptic terminals. This study tested the hypothesis that α2δ-1-bound N-methyl-D-aspartate receptors contribute to presynaptic N-methyl-D-aspartate receptor hyperactivity associated with opioid-induced hyperalgesia and analgesic tolerance.
Methods: Rats (5 mg/kg) and wild-type and α2δ-1-knockout mice (10 mg/kg) were treated intraperitoneally with morphine twice/day for 8 consecutive days, and nociceptive thresholds were examined. Presynaptic N-methyl-D-aspartate receptor activity was recorded in spinal cord slices. Coimmunoprecipitation was performed to examine protein-protein interactions.
Results: Chronic morphine treatment in rats increased α2δ-1 protein amounts in the dorsal root ganglion and spinal cord. Chronic morphine exposure also increased the physical interaction between α2δ-1 and N-methyl-D-aspartate receptors by 1.5 ± 0.3 fold (means ± SD, P = 0.009, n = 6) and the prevalence of α2δ-1-bound N-methyl-D-aspartate receptors at spinal cord synapses. Inhibiting α2δ-1 with gabapentin or genetic knockout of α2δ-1 abolished the increase in presynaptic N-methyl-D-aspartate receptor activity in the spinal dorsal horn induced by morphine treatment. Furthermore, uncoupling the α2δ-1-N-methyl-D-aspartate receptor interaction with an α2δ-1 C terminus-interfering peptide fully reversed morphine-induced tonic activation of N-methyl-D-aspartate receptors at the central terminal of primary afferents. Finally, intraperitoneal injection of gabapentin or intrathecal injection of an α2δ-1 C terminus-interfering peptide or α2δ-1 genetic knockout abolished the mechanical and thermal hyperalgesia induced by chronic morphine exposure and largely preserved morphine's analgesic effect during 8 days of morphine treatment.
Conclusions: α2δ-1-Bound N-methyl-D-aspartate receptors contribute to opioid-induced hyperalgesia and tolerance by augmenting presynaptic N-methyl-D-aspartate receptor expression and activity at the spinal cord level.
Conflict of interest statement
Conflicts of Interest
The authors declare no competing interests with the contents of this study.
Figures


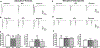

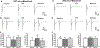

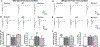


Similar articles
-
α2δ-1-Linked NMDA and AMPA Receptors in Neuropathic Pain and Gabapentinoid Action.J Neurochem. 2025 Apr;169(4):e70064. doi: 10.1111/jnc.70064. J Neurochem. 2025. PMID: 40191897 Review.
-
Brief Opioid Exposure Paradoxically Augments Primary Afferent Input to Spinal Excitatory Neurons via α2δ-1-Dependent Presynaptic NMDA Receptors.J Neurosci. 2022 Dec 14;42(50):9315-9329. doi: 10.1523/JNEUROSCI.1704-22.2022. Epub 2022 Nov 15. J Neurosci. 2022. PMID: 36379705 Free PMC article.
-
mGluR5 from Primary Sensory Neurons Promotes Opioid-Induced Hyperalgesia and Tolerance by Interacting with and Potentiating Synaptic NMDA Receptors.J Neurosci. 2023 Aug 2;43(31):5593-5607. doi: 10.1523/JNEUROSCI.0601-23.2023. Epub 2023 Jul 14. J Neurosci. 2023. PMID: 37451981 Free PMC article.
-
α2δ-1 Upregulation in Primary Sensory Neurons Promotes NMDA Receptor-Mediated Glutamatergic Input in Resiniferatoxin-Induced Neuropathy.J Neurosci. 2021 Jul 7;41(27):5963-5978. doi: 10.1523/JNEUROSCI.0303-21.2021. Epub 2021 Jun 17. J Neurosci. 2021. PMID: 34252037 Free PMC article.
-
Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain.Cell Mol Life Sci. 2019 May;76(10):1889-1899. doi: 10.1007/s00018-019-03047-y. Epub 2019 Feb 20. Cell Mol Life Sci. 2019. PMID: 30788514 Free PMC article. Review.
Cited by
-
α2δ-1-Linked NMDA and AMPA Receptors in Neuropathic Pain and Gabapentinoid Action.J Neurochem. 2025 Apr;169(4):e70064. doi: 10.1111/jnc.70064. J Neurochem. 2025. PMID: 40191897 Review.
-
Quercetin alleviates chronic unpredictable mild stress-induced depression-like behavior by inhibiting NMDAR1 with α2δ-1 in rats.CNS Neurosci Ther. 2024 Apr;30(4):e14724. doi: 10.1111/cns.14724. CNS Neurosci Ther. 2024. PMID: 38615365 Free PMC article.
-
The α2δ-1-NMDA receptor complex and its potential as a therapeutic target for ischemic stroke.Front Neurol. 2023 Apr 20;14:1148697. doi: 10.3389/fneur.2023.1148697. eCollection 2023. Front Neurol. 2023. PMID: 37153659 Free PMC article. Review.
-
Nicotine Motivated Behavior in C. elegans.Int J Mol Sci. 2024 Jan 29;25(3):1634. doi: 10.3390/ijms25031634. Int J Mol Sci. 2024. PMID: 38338915 Free PMC article.
-
The α2δ-1/NMDA receptor complex is involved in brain injury after intracerebral hemorrhage in mice.Ann Clin Transl Neurol. 2021 Jul;8(7):1366-1375. doi: 10.1002/acn3.51372. Epub 2021 May 25. Ann Clin Transl Neurol. 2021. PMID: 34032393 Free PMC article.
References
-
- Ferrini F, Trang T, Mattioli TAM, Laffray S, Del’Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang WB, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y: Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl- homeostasis. Nat Neurosci 2013; 16: 183–192 - PMC - PubMed
-
- Chen SR, Pan HL: Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol 2006; 95: 3086–96 - PubMed
-
- Chen SR, Pan HL: Blocking mu opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res 2006; 1081: 119–25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

