CLINICOPATHOLOGIC CORRELATION OF GEOGRAPHIC ATROPHY SECONDARY TO AGE-RELATED MACULAR DEGENERATION
- PMID: 30839495
- PMCID: PMC6445604
- DOI: 10.1097/IAE.0000000000002461
CLINICOPATHOLOGIC CORRELATION OF GEOGRAPHIC ATROPHY SECONDARY TO AGE-RELATED MACULAR DEGENERATION
Abstract
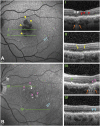
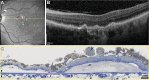
Purpose: In an eye with geographic atrophy (GA) secondary to age-related macular degeneration, we correlated ex vivo histologic features with findings recorded in vivo using optical coherence tomography (OCT), near-infrared reflectance imaging, and fundus autofluorescence.
Methods: In the left eye of an 86-year-old white woman, in vivo near-infrared reflectance and eye-tracked OCT B-scans at each of 6 clinic visits and a baseline fundus autofluorescence image were correlated with high-resolution histologic images of the preserved donor eye.
Results: Clinical imaging showed a small parafoveal multilobular area of GA, subfoveal soft drusen, refractile drusen, hyperreflective lines near the Bruch membrane, subretinal drusenoid deposit (reticular pseudodrusen), and absence of hyperautofluorescent foci at the GA margin. By histology, soft drusen end-stages included avascular fibrosis with highly reflective cholesterol crystals. These accounted for hyperreflective lines near the Bruch membrane in OCT and plaques in near-infrared reflectance imaging. Subretinal drusenoid deposit was thick, continuous, extracellular, extensive outside the fovea, and associated with distinctive retinal pigment epithelium dysmorphia and photoreceptor degeneration. A hyporeflective wedge corresponded to ordered Henle fibers without cellular infiltration. The external limiting membrane descent, which delimits GA, was best visualized in high-quality OCT B-scans. Retinal pigment epithelium and photoreceptor changes at the external limiting membrane descent were consistent with our recent histologic survey of donor eyes.
Conclusion: This case informs on the extent, topography, and lifecycle of extracellular deposits. High-quality OCT scans are required to reveal all tissue features relevant to age-related macular degeneration progression to GA, especially the external limiting membrane descent. Histologically validated signatures of structural OCT B-scans can serve as references for other imaging modalities.
Conflict of interest statement
None of the authors has any financial/conflicting interests to disclose.
Figures







Similar articles
-
Subretinal drusenoid deposits AKA pseudodrusen.Surv Ophthalmol. 2018 Nov-Dec;63(6):782-815. doi: 10.1016/j.survophthal.2018.05.005. Epub 2018 May 31. Surv Ophthalmol. 2018. PMID: 29859199 Review.
-
Histologic and Optical Coherence Tomographic Correlates in Drusenoid Pigment Epithelium Detachment in Age-Related Macular Degeneration.Ophthalmology. 2017 May;124(5):644-656. doi: 10.1016/j.ophtha.2016.12.034. Epub 2017 Jan 30. Ophthalmology. 2017. PMID: 28153442 Free PMC article.
-
Stages of Drusen-Associated Atrophy in Age-Related Macular Degeneration Visible via Histologically Validated Fundus Autofluorescence.Ophthalmol Retina. 2021 Aug;5(8):730-742. doi: 10.1016/j.oret.2020.11.006. Epub 2020 Nov 18. Ophthalmol Retina. 2021. PMID: 33217617 Free PMC article.
-
Optical Coherence Tomography Reflective Drusen Substructures Predict Progression to Geographic Atrophy in Age-related Macular Degeneration.Ophthalmology. 2016 Dec;123(12):2554-2570. doi: 10.1016/j.ophtha.2016.08.047. Epub 2016 Oct 25. Ophthalmology. 2016. PMID: 27793356 Free PMC article.
-
Perspectives on reticular pseudodrusen in age-related macular degeneration.Surv Ophthalmol. 2016 Sep-Oct;61(5):521-37. doi: 10.1016/j.survophthal.2016.02.005. Epub 2016 Mar 17. Surv Ophthalmol. 2016. PMID: 26994868 Review.
Cited by
-
Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 4.Ophthalmology. 2020 Mar;127(3):394-409. doi: 10.1016/j.ophtha.2019.09.035. Epub 2019 Sep 30. Ophthalmology. 2020. PMID: 31708275 Free PMC article.
-
Longitudinal Changes of Visual Sensitivity With Geographic Atrophy Progression Assessed by Defect-Mapping Microperimetry.Invest Ophthalmol Vis Sci. 2024 Dec 2;65(14):13. doi: 10.1167/iovs.65.14.13. Invest Ophthalmol Vis Sci. 2024. PMID: 39636724 Free PMC article.
-
[Correlation of in vivo/ex vivo imaging of the posterior eye segment].Ophthalmologe. 2020 Dec;117(12):1188-1195. doi: 10.1007/s00347-020-01228-w. Ophthalmologe. 2020. PMID: 32989527 Free PMC article. Review.
-
Measuring the Contributions of Basal Laminar Deposit and Bruch's Membrane in Age-Related Macular Degeneration.Invest Ophthalmol Vis Sci. 2020 Nov 2;61(13):19. doi: 10.1167/iovs.61.13.19. Invest Ophthalmol Vis Sci. 2020. PMID: 33186466 Free PMC article.
-
Customized Evaluation of Progressive Visual Sensitivity Loss in Geographic Atrophy to Improve the Power of Clinical Trials.Ophthalmol Sci. 2025 Mar 14;5(4):100763. doi: 10.1016/j.xops.2025.100763. eCollection 2025 Jul-Aug. Ophthalmol Sci. 2025. PMID: 40276124 Free PMC article.
References
-
- Jaffe GJ, Schmitz-Valckenberg S, Boyer D, et al. Randomized trial to evaluate tandospirone in geographic atrophy secondary to age-related macular degeneration: the GATE study. Am J Ophthalmol 2015;160:1226–1234. - PubMed
-
- Rosenfeld PJ, Dugel PU, Holz FG, et al. Emixustat hydrochloride for geographic atrophy secondary to age-related macular degeneration: a randomized clinical trial. Ophthalmology 2018;125:1556–1567. - PubMed
-
- Holz FG, Sadda SR, Staurenghi G, et al. Imaging protocols in clinical studies in advanced age-related macular degeneration: recommendations from classification of atrophy consensus meetings. Ophthalmology 2017;124:464–478. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

