Preparation of nanoporous BiVO4/TiO2/Ti film through electrodeposition for photoelectrochemical water splitting
- PMID: 30839769
- PMCID: PMC6170590
- DOI: 10.1098/rsos.180728
Preparation of nanoporous BiVO4/TiO2/Ti film through electrodeposition for photoelectrochemical water splitting
Abstract
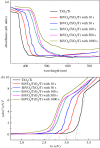
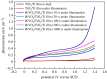
A nanoporous BiVO4/TiO2/Ti film was successfully fabricated by electrodepositing a nanoporous BiOI film on nanoporous TiO2 arrays followed by annealing at 450°C for 2 h. The electrodeposition of BiOI film was carried out at different times (10, 30, 100, 500 and 1000 s) in Bi(NO3)3 and KI solution. The morphological, crystallographic and photoelectrochemical properties of the prepared BiVO4/TiO2/Ti heterojunction film were examined by using different characterization techniques. UV-vis spectrum absorption studies confirmed an increase in absorption intensities with increasing electrodeposition time, and the band gap of BiVO4/TiO2/Ti film is lower than that of TiO2/Ti. The photocatalytic efficiency of BiVO4/TiO2/Ti heterojunction film was higher compared to that of the TiO2/Ti film owing to the longer transient decay time for BiVO4/TiO2/Ti film (3.2 s) than that of TiO2/Ti film (0.95 s) in our experiment. The BiVO4/TiO2/Ti heterojunction film prepared by electrodeposition for 1000 s followed by annealing showed a high photocurrent density of 0.3363 mA cm-2 at 0.6 V versus saturated calomel electrode. Furthermore, the lowest charge transfer resistance from electrochemical impedance spectroscopy was recorded for the BiVO4/TiO2/Ti film (1000 s) under irradiation.
Keywords: BiVO4/TiO2/Ti photoelectrodes; electrodeposition; photocurrent density; water splitting.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Jaegermann W, Tributsch H. 1988. Interfacial properties of semiconducting transition metal chalcogenides. Prog. Surf. Sci. 29, 1–167. ( 10.1016/0079-6816(88)90015-9) - DOI
-
- Yan K, Qiu Y, Xiao S, Gong J, Zhao S, Xu J, Meng X, Yang S, Xu J. 2017. Self-driven hematite-based photoelectrochemical water splitting cells with three-dimensional nanobowl heterojunction and high-photovoltage perovskite solar cells. Mater. Today Energy 6, 128–135. ( 10.1016/j.mtener.2017.09.006) - DOI
-
- Sun T, Cui D, Ma Q, Peng X, Yuan L. 2017. Synthesis of BiVO4/MWCNT/Ag@AgCl composite with enhanced photocatalytic performance. J. Phys. Chem. Solids 111, 190–198. ( 10.1016/j.jpcs.2017.08.006) - DOI
-
- Zhang K, Liu Y, Deng J, Xie S, Dai H. 2017. Fe2O3/3DOM BiVO4: High-performance photocatalysts for the visible light-driven degradation of 4-nitrophenol. Appl. Catal. B 202, 569–579. ( 10.1016/j.apcatb.2016.09.069) - DOI
-
- Baek JH, Kim BJ, Han GS, Hwang SW, Kim DR, Cho IS, Jung HS. 2017. BiVO4/WO3/SnO2 double-heterojunction photoanode with enhanced charge separation and visible-transparency for bias-free solar water-splitting with a perovskite solar cell. ACS Appl. Mater. Interfaces 9, 1479–1487. ( 10.1021/acsami.6b12782) - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
