Development and Validation of a Risk Prediction Model for In-Hospital Mortality Among Patients With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis-ABCD-10
- PMID: 30840032
- PMCID: PMC6459085
- DOI: 10.1001/jamadermatol.2018.5605
Development and Validation of a Risk Prediction Model for In-Hospital Mortality Among Patients With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis-ABCD-10
Erratum in
-
Error in P Value Report.JAMA Dermatol. 2019 Sep 1;155(9):1090. doi: 10.1001/jamadermatol.2019.2200. JAMA Dermatol. 2019. PMID: 31365029 Free PMC article. No abstract available.
-
Error in Author's Name.JAMA Dermatol. 2019 Sep 1;155(9):1090. doi: 10.1001/jamadermatol.2019.2835. JAMA Dermatol. 2019. PMID: 31509161 Free PMC article. No abstract available.
Abstract
Importance: Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) is a spectrum of severe mucocutaneous drug reaction associated with significant morbidity and mortality. A previously developed SJS/TEN-specific severity-of-illness model (Score of Toxic Epidermal Necrolysis [SCORTEN]) has been reported to overestimate and underestimate SJS/TEN-related in-hospital mortality in various populations.
Objective: To derive a risk prediction model for in-hospital mortality among patients with SJS/TEN and to compare prognostic accuracy with the SCORTEN model in a multi-institutional cohort of patients in the United States.
Design, setting, and participants: Data from a multicenter cohort of patients 18 years and older treated for SJS/TEN between January 1, 2000, and June 1, 2015, were obtained from inpatient consult databases and electronic medical record systems at 18 medical centers in the United States as part of the Society for Dermatology Hospitalists. A risk model was derived based on data from 370 of these patients. Model discrimination (calculated as area under the receiver operating characteristic curve [AUC]) and calibration (calculated as predicted vs observed mortality, and examined using the Hosmer-Lemeshow goodness-of-fit statistic) were assessed, and the predictive accuracy was compared with that of SCORTEN. All analysis took place between December 2016 and April 2018.
Main outcomes and measures: In-hospital mortality.
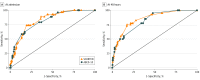
Results: Among 370 patients (mean [SD] age 49.0 [19.1] years; 195 [52.7%] women), 54 (15.14%) did not survive to hospital discharge. Five covariates, measured at the time of admission, were independent predictors of in-hospital mortality: age in years (odds ratio [OR], 1.05; 95% CI, 1.02-1.07), body surface area (BSA) in percentage of epidermal detachment (OR, 1.02; 95% CI, 1.01-1.04), serum bicarbonate level below 20 mmol/L (OR, 2.90; 95% CI, 1.43-5.88), active cancer (OR, 4.40; 95% CI, 1.82-10.61), and dialysis prior to admission (OR, 15.94; 95% CI, 3.38-66.30). A severity-of-illness score was calculated by taking the sum of 1 point each for age 50 years or older, epidermal detachment greater than 10% of BSA, and serum bicarbonate level below 20 mmol/L; 2 points for the presence of active cancer; and 3 points for dialysis prior to admission. The score was named ABCD-10 (age, bicarbonate, cancer, dialysis, 10% BSA). The ABCD-10 model showed good discrimination (AUC, 0.816; 95% CI, 0.759-0.872) and calibration (Hosmer-Lemeshow goodness of fit test, P = .30). For SCORTEN, on admission, the AUC was 0.827 (95% CI, 0.774-0.879) and was not significantly different from that of the ABCD-10 model (P = .72).
Conclusions and relevance: In this cohort of patients with SJS/TEN, ABCD-10 accurately predicted in-hospital mortality, with discrimination that was not significantly different from SCORTEN. Additional research is needed to validate ABCD-10 in other populations. Future use of a new mortality prediction model may provide improved prognostic information for contemporary patients, including those enrolled in observational studies and therapeutic trials.
Conflict of interest statement
Figures
References
-
- Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58(1):33-40. doi: 10.1016/j.jaad.2007.08.039 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

