Neuronal autoantibodies associated with cognitive impairment in melanoma patients
- PMID: 30840061
- PMCID: PMC6551450
- DOI: 10.1093/annonc/mdz083
Neuronal autoantibodies associated with cognitive impairment in melanoma patients
Abstract
Background: Cancer-related cognitive impairment is an important complication in cancer patients, yet the underlying mechanisms remain unknown. Over the last decade, the field of paraneoplastic neurological syndromes has been dramatically changed by the discovery of new neuronal autoantibodies, some of them associated with cognitive impairment. We aimed to assess the prevalence of neuronal autoantibodies in melanoma patients and their association with neurological and cognitive dysfunction.
Patients and methods: A total of 157 consecutive melanoma patients with a median age of 63 years were recruited at the Department of Dermatology, Charité-Universitätsmedizin Berlin and tested for neuronal autoantibodies. A comprehensive neuropsychological assessment was carried out in a selected subgroup of 84 patients after exclusion of patients with confounding factors for a cognitive dysfunction, including brain metastases, relevant medication, and neurological disorders.
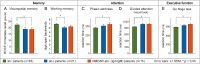
Results: Neuronal autoantibodies were found in 22.3% of melanoma patients. The most frequent antibodies were IgA/IgM anti-NMDAR antibodies. Applying the International Cognition and Cancer Task Force criteria, 36.9% had cognitive impairment, however, with a threefold higher odds in antibody-positive compared with antibody-negative patients (57.1% versus 30.2%, OR = 3.1, 95% CI: 1.1 to 8.6; P = 0.037). In patients with anti-NMDAR antibodies, this impairment increased with higher antibody titers (P = 0.007). Antibody-positive patients had a significantly impaired overall cognitive performance (z-value: -0.38 ± 0.69 versus 0.00 ± 0.56; P = 0.014) as well as significant impairments in tests of memory, attention, and executive function. In a multiple linear regression analysis, autoantibodies were an independent risk factor for cognitive impairment (B = -0.282; 95% CI: -0.492 to -0.071; P = 0.009). Autoantibody seropositivity was associated with immune checkpoint inhibitor treatment and a history of autoimmune diseases.
Conclusions: A large number of melanoma patients harbor neuronal autoantibodies that are associated with significant cognitive impairment affecting memory, attention, and executive function. Neuronal autoantibodies might represent a pathophysiological factor and possible biomarker in the development of cancer-related cognitive impairment.
Keywords: cancer-related cognitive impairment; immune checkpoint inhibitor; melanoma; neuronal autoantibodies; paraneoplastic neurological syndromes.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures


References
-
- Giometto B, Grisold W, Vitaliani R. et al. Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch Neurol 2010; 67(3): 330–335. - PubMed
-
- Jarius S, Steinmeyer F, Knobel A. et al. GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J Neuroimmunol 2013; 256(1–2): 94–96. - PubMed
-
- Becquart C, Ryckewaert G, Desmedt E. et al. Limbic encephalitis: a new paraneoplastic auto-immune manifestation associated with metastatic melanoma? Ann Dermatol Venereol 2013; 140(4): 278–281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

