Physiologically Based Pharmacokinetic Modeling for Trimethoprim and Sulfamethoxazole in Children
- PMID: 30840200
- PMCID: PMC6586496
- DOI: 10.1007/s40262-018-00733-1
Physiologically Based Pharmacokinetic Modeling for Trimethoprim and Sulfamethoxazole in Children
Abstract
Objective: The aims of this study were to (1) determine whether opportunistically collected data can be used to develop physiologically based pharmacokinetic (PBPK) models in pediatric patients; and (2) characterize age-related maturational changes in drug disposition for the renally eliminated and hepatically metabolized antibiotic trimethoprim (TMP)-sulfamethoxazole (SMX).
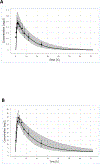
Methods: We developed separate population PBPK models for TMP and SMX in children after oral administration of the combined TMP-SMX product and used sparse and opportunistically collected plasma concentration samples to validate our pediatric model. We evaluated predictability of the pediatric PBPK model based on the number of observed pediatric data out of the 90% prediction interval. We performed dosing simulations to target organ and tissue (skin) concentrations greater than the methicillin-resistant Staphylococcus aureus (MRSA) minimum inhibitory concentration (TMP 2 mg/L; SMX 9.5 mg/L) for at least 50% of the dosing interval.
Results: We found 67-87% and 71-91% of the observed data for TMP and SMX, respectively, were captured within the 90% prediction interval across five age groups, suggesting adequate fit of our model. Our model-rederived optimal dosing of TMP at the target tissue was in the range of recommended dosing for TMP-SMX in children in all age groups by current guidelines for the treatment of MRSA.
Conclusion: We successfully developed a pediatric PBPK model of the combination antibiotic TMP-SMX using sparse and opportunistic pediatric pharmacokinetic samples. This novel and efficient approach has the potential to expand the use of PBPK modeling in pediatric drug development.
Conflict of interest statement
Conflicts of interest:
None of the other authors have anything to disclose.
Figures



References
-
- Alonso Campero R, Bernardo Escudero R, Del Cisne Valle Alvarez D, González de la Parra M, Namur Montalvo S, Burke Fraga V, et al. Bioequivalence of two commercial preparations of trimethoprim/sulfamethoxazole: a randomized, single-dose, single-blind, crossover trial. Clin Ther. 2007;29:326–33. - PubMed
-
- Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm. 2004;58:265–78. - PubMed
-
- Schwartz DE, Zeigler WH. Assay and pharmacokinetics of trimethoprim in man and animals. Postgrad Med J. 1969;45:Supp 32–7. - PubMed
-
- Karpman E, Kurzrock EA. Adverse reactions of nitrofurantoin, trimethoprim and sulfamethoxazole in children. J Urol. 2004;172:448–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

