Targeting Burkitt lymphoma with a tumor cell-specific heptamethine carbocyanine-cisplatin conjugate
- PMID: 30840322
- PMCID: PMC6618854
- DOI: 10.1002/cncr.32033
Targeting Burkitt lymphoma with a tumor cell-specific heptamethine carbocyanine-cisplatin conjugate
Abstract
Background: Burkitt lymphoma is a fast-growing mature B cell malignancy, whose genetic hallmark is translocation and activation of the c-myc gene. Prompt multiagent immunochemotherapy regimens can have favorable outcomes, but prognosis is poor in refractory or relapsed disease. We previously identified a novel family of near-infrared heptamethine carbocyanine fluorescent dyes (HMCD or DZ) with tumor-homing properties via organic anion-transporting peptides. These membrane carriers have uptake in tumor cells but not normal cells in cell culture, mouse and dog tumor models, patient-derived xenografts, and perfused kidney cancers in human patients.
Methods: Here we report the cytotoxic effects of a synthesized conjugate of DZ with cisplatin (CIS) on B cell lymphoma CA46, Daudi, Namalwa, Raji, and Ramos cell lines in cell culture and in xenograft tumor formation. Impaired mitochondrial membrane permeability was examined as the mechanism of DZ-CIS-induced lymphoma cell death.
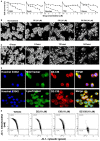
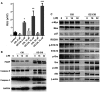
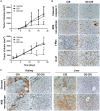
Results: The new conjugate, DZ-CIS, is cytotoxic against Burkitt lymphoma cell lines and tumor models. DZ-CIS retains tumor-homing properties to mitochondrial and lysosomal compartments, does not accumulate in normal cells and tissues, and has no nephrotoxicity in mice. DZ-CIS accumulated in Burkitt lymphoma cells and tumors induces apoptosis and retards tumor cell growth in culture and xenograft tumor growth in mice.
Conclusion: DZ-CIS downregulated c-myc and overcame CIS resistance in myc-driven TP53-mutated aggressive B cell Burkitt lymphoma. We propose that DZ-CIS could be used to treat relapsed/refractory aggressive Burkitt lymphomas.
Keywords: Burkitt lymphoma; cell death; cisplatin; conjugate; heptamethine carbocyanine.
© 2019 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Conflict of interest statement
DZ‐CIS is licensed to DaZen Theranostics, Inc., where Leland W. K. Chung is currently serving as the Chairman of the Board and Chief Scientist for the development of one of the licensed lead compounds from laboratory to the clinic and for which Stefan Mrdenovic, Yi Zhang, Ruoxiang Wang, and Gina Chia‐Yi Chu are shareholders. Leland W. K. Chung also has a patent pending (US 2016/0310604 A1).
Figures
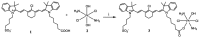

References
-
- Molyneux EM, Rochford R, Griffin B, et al. Burkitt’s lymphoma. Lancet. 2012;379:1234‐1244. - PubMed
-
- Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumors of haematopoietic and lymphoid tissues, Revised Fourth Edition. Geneva, Switzerland: World Health Organization; 2017.
-
- Brown JR, Freedman AS, Aster JC. Pathobiology of Burkitt lymphoma. UpToDate. https://www.uptodate.com/contents/pathobiology-of-burkitt-lymphoma. Accessed July 1, 2018.
-
- ar‐Rushdi A, Nishikura K, Erikson J, Watt R, Rovera G, Croce CM. Differential expression of the translocated and the untranslocated c‐myc oncogene in Burkitt lymphoma. Science. 1983;222:390‐393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

