Basolateral amygdala neurons are activated during threat expectation
- PMID: 30840520
- PMCID: PMC6589710
- DOI: 10.1152/jn.00807.2018
Basolateral amygdala neurons are activated during threat expectation
Abstract
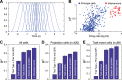
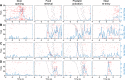
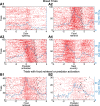
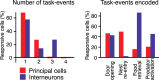
Fear conditioning studies have led to the view that the amygdala contains neurons that signal threat and in turn elicit defensive behaviors through their brain stem and hypothalamic targets. In agreement with this model, a prior unit-recording study in rats performing a seminaturalistic foraging task revealed that many lateral amygdala (LA) neurons are predator responsive. In contrast, our previous study emphasized that most basolateral (BL) amygdala neurons are inhibited at proximity of the predator. However, the two studies used different methods to analyze unit activity, complicating comparisons between them. By applying the same method to the sample of BL neurons we recorded previously, the present study revealed that most principal cells are inhibited by the predator and only 4.5% are activated. Moreover, two-thirds of these cells were also activated by nonthreatening stimuli. In fact, fitting unit activity with a generalized linear model revealed that the only task variables associated with a prevalent positive modulation of BL activity were expectation of the predator's presence and whether the prior trial had been a failure or success. At odds with the threat-coding model of the amygdala, actual confrontation with the predator was usually associated with a widespread inhibition of principal BL neurons. NEW & NOTEWORTHY The basolateral amygdala (BL) is thought to contain neurons that signal threat, in turn eliciting defensive behaviors. In contrast, the present study reports that very few principal BL cells are responsive to threats and that most of them are also activated by nonthreatening stimuli. Yet, expectation of the threat's presence was associated with a prevalent positive modulation of BL activity; actual confrontation with the threat was associated with a widespread inhibition.
Keywords: amygdala; fear; foraging; predator; threat.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

