Postural control of a musculoskeletal model against multidirectional support surface translations
- PMID: 30840650
- PMCID: PMC6402659
- DOI: 10.1371/journal.pone.0212613
Postural control of a musculoskeletal model against multidirectional support surface translations
Abstract
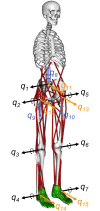
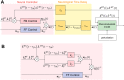
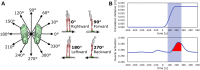
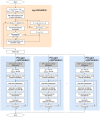
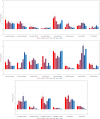
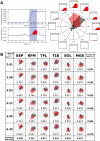
The human body is a complex system driven by hundreds of muscles, and its control mechanisms are not sufficiently understood. To understand the mechanisms of human postural control, neural controller models have been proposed by different research groups, including our feed-forward and feedback control model. However, these models have been evaluated under forward and backward perturbations, at most. Because a human body experiences perturbations from many different directions in daily life, neural controller models should be evaluated in response to multidirectional perturbations, including in the forward/backward, lateral, and diagonal directions. The objective of this study was to investigate the validity of an NC model with FF and FB control under multidirectional perturbations. We developed a musculoskeletal model with 70 muscles and 15 degrees of freedom of joints, positioned it in a standing posture by using the neural controller model, and translated its support surface in multiple directions as perturbations. We successfully determined the parameters of the neural controller model required to maintain the stance of the musculoskeletal model for each perturbation direction. The trends in muscle response magnitudes and the magnitude of passive ankle stiffness were consistent with the results of experimental studies. We conclude that the neural controller model can adapt to multidirectional perturbations by generating suitable muscle activations. We anticipate that the neural controller model could be applied to the study of the control mechanisms of patients with torso tilt and diagnosis of the change in control mechanisms from patients' behaviors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
EMG responses to maintain stance during multidirectional surface translations.J Neurophysiol. 1998 Oct;80(4):1939-50. doi: 10.1152/jn.1998.80.4.1939. J Neurophysiol. 1998. PMID: 9772251
-
Investigation of the effect of tonus on the change in postural control strategy using musculoskeletal simulation.Gait Posture. 2020 Feb;76:298-304. doi: 10.1016/j.gaitpost.2019.12.015. Epub 2019 Dec 16. Gait Posture. 2020. PMID: 31884257
-
Triggering of balance corrections and compensatory strategies in a patient with total leg proprioceptive loss.Exp Brain Res. 2002 Jan;142(1):91-107. doi: 10.1007/s00221-001-0926-3. Epub 2001 Nov 14. Exp Brain Res. 2002. PMID: 11797087
-
Vestibular influences on human postural control in combinations of pitch and roll planes reveal differences in spatiotemporal processing.Exp Brain Res. 2001 Sep;140(1):95-111. doi: 10.1007/s002210100802. Exp Brain Res. 2001. PMID: 11500802
-
The Role of Predictability of Perturbation in Control of Posture: A Scoping Review.Motor Control. 2022 Jan 1;26(1):97-143. doi: 10.1123/mc.2021-0074. Epub 2021 Dec 10. Motor Control. 2022. PMID: 34891127
Cited by
-
Characterization of Human Balance through a Reinforcement Learning-based Muscle Controller.PLoS One. 2025 Apr 1;20(4):e0320211. doi: 10.1371/journal.pone.0320211. eCollection 2025. PLoS One. 2025. PMID: 40168263 Free PMC article.
-
A sensorimotor enhanced neuromusculoskeletal model for simulating postural control of upright standing.Front Neurosci. 2024 May 15;18:1393749. doi: 10.3389/fnins.2024.1393749. eCollection 2024. Front Neurosci. 2024. PMID: 38812972 Free PMC article.
-
Methods for integrating postural control into biomechanical human simulations: a systematic review.J Neuroeng Rehabil. 2023 Aug 21;20(1):111. doi: 10.1186/s12984-023-01235-3. J Neuroeng Rehabil. 2023. PMID: 37605197 Free PMC article.
-
Optimal controllers resembling postural sway during upright stance.PLoS One. 2023 May 2;18(5):e0285098. doi: 10.1371/journal.pone.0285098. eCollection 2023. PLoS One. 2023. PMID: 37130115 Free PMC article.
-
A Neural Controller Model Considering the Vestibulospinal Tract in Human Postural Control.Front Comput Neurosci. 2022 Feb 25;16:785099. doi: 10.3389/fncom.2022.785099. eCollection 2022. Front Comput Neurosci. 2022. PMID: 35283745 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

