FBXO7 sensitivity of phenotypic traits elucidated by a hypomorphic allele
- PMID: 30840666
- PMCID: PMC6402633
- DOI: 10.1371/journal.pone.0212481
FBXO7 sensitivity of phenotypic traits elucidated by a hypomorphic allele
Abstract
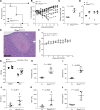
FBXO7 encodes an F box containing protein that interacts with multiple partners to facilitate numerous cellular processes and has a canonical role as part of an SCF E3 ubiquitin ligase complex. Mutation of FBXO7 is responsible for an early onset Parkinsonian pyramidal syndrome and genome-wide association studies have linked variants in FBXO7 to erythroid traits. A putative orthologue in Drosophila, nutcracker, has been shown to regulate the proteasome, and deficiency of nutcracker results in male infertility. Therefore, we reasoned that modulating Fbxo7 levels in a murine model could provide insights into the role of this protein in mammals. We used a targeted gene trap model which retained 4-16% residual gene expression and assessed the sensitivity of phenotypic traits to gene dosage. Fbxo7 hypomorphs showed regenerative anaemia associated with a shorter erythrocyte half-life, and male mice were infertile. Alterations to T cell phenotypes were also observed, which intriguingly were both T cell intrinsic and extrinsic. Hypomorphic mice were also sensitive to infection with Salmonella, succumbing to a normally sublethal challenge. Despite these phenotypes, Fbxo7 hypomorphs were produced at a normal Mendelian ratio with a normal lifespan and no evidence of neurological symptoms. These data suggest that erythrocyte survival, T cell development and spermatogenesis are particularly sensitive to Fbxo7 gene dosage.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

