Trio Haploinsufficiency Causes Neurodevelopmental Disease-Associated Deficits
- PMID: 30840899
- PMCID: PMC6436967
- DOI: 10.1016/j.celrep.2019.02.022
Trio Haploinsufficiency Causes Neurodevelopmental Disease-Associated Deficits
Abstract
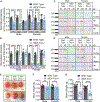
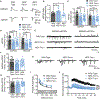
Heterozygous coding mutations in TRIO are associated with neurodevelopmental disorders, including autism, schizophrenia, bipolar disorder, and epilepsy, and impair TRIO's biochemical activities. To model mutant alleles, we ablated one or both Trio alleles from excitatory neurons in the cortex and hippocampus of mice. Trio haploinsufficiency increases anxiety and impairs social preference and motor coordination. Trio loss reduces forebrain size and dendritic arborization but increases dendritic spine densities. Cortical synapses in Trio haploinsufficient mice are small, exhibit pre- and postsynaptic deficits, and cannot undergo long-term potentiation. Similar phenotypes are observed in Trio knockout mice. Overall, Trio haploinsufficiency causes severe disease-relevant deficits in behavior and neuronal structure and function. Interestingly, phosphodiesterase 4A5 (PDE4A5) levels are reduced and protein kinase A (PKA) signaling is increased when TRIO levels are reduced. Elevation of PDE4A5 and drug-based attenuation of PKA signaling rescue Trio haploinsufficiency-related dendritic spine defects, suggesting an avenue for therapeutic intervention for TRIO-related neurodevelopmental disorders.
Keywords: TRIO; anxiety; dendritic spine; haploinsufficiency; long-term potentiation; motor cortex; neurodevelopmental disorder; phosphodiesterase 4A5; social preference; triple functional domain protein.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures





References
-
- Awasaki T, Saito M, Sone M, Suzuki E, Sakai R, Ito K, and Hama C (2000). The Drosophila Trio plays an essential role in patterning of axons by regulating their directional extension. Neuron 26, 119–131. - PubMed
-
- Ba W, Yan Y, Reijnders MRF, Schuurs–Hoeijmakers JHM, Feenstra I, Bongers EMHF, Bosch DGM, De Leeuw N, Pfundt R, Gilissen C, et al. (2016). TRIO loss of function is associated with mild intellectual disability and affects dendritic branching and synapse function. Hum. Mol. Genet 25, 892–902. - PMC - PubMed
-
- Ball RW, Warren–Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, and Haghighi AP (2010). Retrograde BMP signaling controls synaptic growth at the NMJ by regulating Trio expression in motor neurons. Neuron 66, 536–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

