Spatial and Spectral Mapping and Decomposition of Neural Dynamics and Organization of the Mouse Brain with Multispectral Optoacoustic Tomography
- PMID: 30840901
- PMCID: PMC6403416
- DOI: 10.1016/j.celrep.2019.02.020
Spatial and Spectral Mapping and Decomposition of Neural Dynamics and Organization of the Mouse Brain with Multispectral Optoacoustic Tomography
Abstract
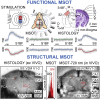
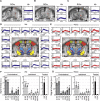
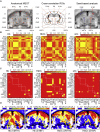
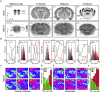
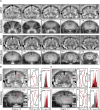
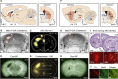
In traditional optical imaging, limited light penetration constrains high-resolution interrogation to tissue surfaces. Optoacoustic imaging combines the superb contrast of optical imaging with deep penetration of ultrasound, enabling a range of new applications. We used multispectral optoacoustic tomography (MSOT) for functional and structural neuroimaging in mice at resolution, depth, and specificity unattainable by other neuroimaging modalities. Based on multispectral readouts, we computed hemoglobin gradient and oxygen saturation changes related to processing of somatosensory signals in different structures along the entire subcortical-cortical axis. Using temporal correlation analysis and seed-based maps, we reveal the connectivity between cortical, thalamic, and sub-thalamic formations. With the same modality, high-resolution structural tomography of intact mouse brain was achieved based on endogenous contrasts, demonstrating near-perfect matches with anatomical features revealed by histology. These results extend the limits of noninvasive observations beyond the reach of standard high-resolution neuroimaging, verifying the suitability of MSOT for small-animal studies.
Keywords: hemodynamic response; label-free interrogation; near-infrared neuroimaging; photoacoustic imaging; temporal coherence; whole-brain tomography.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Barden H., Levine S. Histochemical observations on rodent brain melanin. Brain Res. Bull. 1983;10:847–851. - PubMed
-
- Berger T., Borgdorff A., Crochet S., Neubauer F.B., Lefort S., Fauvet B., Ferezou I., Carleton A., Lüscher H.R., Petersen C.C. Combined voltage and calcium epifluorescence imaging in vitro and in vivo reveals subthreshold and suprathreshold dynamics of mouse barrel cortex. J. Neurophysiol. 2007;97:3751–3762. - PubMed
-
- Boas D.A., Dale A.M., Franceschini M.A. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage. 2004;23(Suppl 1):S275–S288. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

