Hemodynamic Stimulation Using the Biomimetic Cardiac Tissue Model (BCTM) Enhances Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes
- PMID: 30840966
- PMCID: PMC7765732
- DOI: 10.1159/000496934
Hemodynamic Stimulation Using the Biomimetic Cardiac Tissue Model (BCTM) Enhances Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes
Abstract
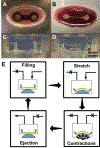
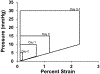
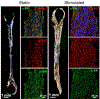
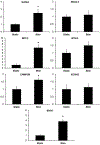
Human induced pluripotent stem cell (hiPSC)-derived cardio-myocytes (hiPSC-CMs) hold great promise for cardiovascular disease modeling and regenerative medicine. However, these cells are both structurally and functionally -immature, primarily due to their differentiation into cardiomyocytes occurring under static culture which only reproduces biomolecular cues and ignores the dynamic hemo-dynamic cues that shape early and late heart development during cardiogenesis. To evaluate the effects of hemodynamic stimuli on hiPSC-CM maturation, we used the biomimetic cardiac tissue model to reproduce the hemodynamics and pressure/volume changes associated with heart development. Following 7 days of gradually increasing stimulation, we show that hemodynamic loading results in (a) enhanced alignment of the cells and extracellular matrix, (b) significant increases in genes associated with physiological hypertrophy, (c) noticeable changes in sarcomeric organization and potential changes to cellular metabolism, and (d) a significant increase in fractional shortening, suggestive of a positive force frequency response. These findings suggest that culture of hiPSC-CMs under conditions that accurately reproduce hemodynamic cues results in structural orga-nization and molecular signaling consistent with organ growth and functional maturation.
Keywords: Cardiovascular tissue engineering; Hemodynamics; Hypertrophy; Stem cell differentiation.
© 2019 S. Karger AG, Basel.
Conflict of interest statement
Disclosure Statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Biomimetic Cardiac Tissue Model Enables the Adaption of Human Induced Pluripotent Stem Cell Cardiomyocytes to Physiological Hemodynamic Loads.Anal Chem. 2016 Oct 4;88(19):9862-9868. doi: 10.1021/acs.analchem.6b03105. Epub 2016 Sep 23. Anal Chem. 2016. PMID: 27620367 Free PMC article.
-
Enhanced structural maturation of human induced pluripotent stem cell-derived cardiomyocytes under a controlled microenvironment in a microfluidic system.Acta Biomater. 2020 Jan 15;102:273-286. doi: 10.1016/j.actbio.2019.11.044. Epub 2019 Nov 26. Acta Biomater. 2020. PMID: 31778832
-
Maturation of Pluripotent Stem Cell-Derived Cardiomyocytes Enables Modeling of Human Hypertrophic Cardiomyopathy.Stem Cell Reports. 2021 Mar 9;16(3):519-533. doi: 10.1016/j.stemcr.2021.01.018. Epub 2021 Feb 25. Stem Cell Reports. 2021. PMID: 33636116 Free PMC article.
-
Modeling Cardiovascular Diseases with hiPSC-Derived Cardiomyocytes in 2D and 3D Cultures.Int J Mol Sci. 2020 May 11;21(9):3404. doi: 10.3390/ijms21093404. Int J Mol Sci. 2020. PMID: 32403456 Free PMC article. Review.
-
Biowire platform for maturation of human pluripotent stem cell-derived cardiomyocytes.Methods. 2016 May 15;101:21-6. doi: 10.1016/j.ymeth.2015.11.005. Epub 2015 Nov 4. Methods. 2016. PMID: 26546730 Review.
Cited by
-
Architecture design and advanced manufacturing of heart-on-a-chip: scaffolds, stimulation and sensors.Microsyst Nanoeng. 2024 Jul 11;10:96. doi: 10.1038/s41378-024-00692-7. eCollection 2024. Microsyst Nanoeng. 2024. PMID: 39006908 Free PMC article. Review.
-
Biomimetic cardiac tissue culture model (CTCM) to emulate cardiac physiology and pathophysiology ex vivo.Commun Biol. 2022 Sep 9;5(1):934. doi: 10.1038/s42003-022-03919-3. Commun Biol. 2022. PMID: 36085302 Free PMC article.
-
Tissue-in-a-Tube: three-dimensional in vitro tissue constructs with integrated multimodal environmental stimulation.Mater Today Bio. 2020 Jul 28;7:100070. doi: 10.1016/j.mtbio.2020.100070. eCollection 2020 Jun. Mater Today Bio. 2020. PMID: 32875285 Free PMC article.
-
Tissue Chips and Microphysiological Systems for Disease Modeling and Drug Testing.Micromachines (Basel). 2021 Jan 28;12(2):139. doi: 10.3390/mi12020139. Micromachines (Basel). 2021. PMID: 33525451 Free PMC article. Review.
-
Bioengineering approaches to mature induced pluripotent stem cell-derived atrial cardiomyocytes to model atrial fibrillation.Exp Biol Med (Maywood). 2021 Aug;246(16):1816-1828. doi: 10.1177/15353702211009146. Epub 2021 Apr 25. Exp Biol Med (Maywood). 2021. PMID: 33899540 Free PMC article. Review.
References
-
- 2017. “Cardiovascular diseases (CVDs).” World Health Organization, Last Modified 5/17/2017, accessed 6/5/2018 http://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseas....
-
- Bellin Milena, Marchetto Maria C, Gage Fred H, and Mummery Christine L. 2012. “Induced pluripotent stem cells: the new patient?” Nature reviews Molecular cell biology 13 (11):713. - PubMed
-
- Bénardeau Agnès, Hatem Stéphane N., Rücker-Martin Catherine, Tessier Sophie, Dinanian Sylvie, Samuel Jane-Lyse, Coraboeuf Edouard, and Mercadier Jean-Jaques. 1997. “Primary Culture of Human Atrial Myocytes is Associated with the Appearance of Structural and Functional Characteristics of Immature Myocardium.” Journal of Molecular and Cellular Cardiology 29 (5):1307–1320. doi: 10.1006/jmcc.1996.0366. - DOI - PubMed
-
- Benjamin Emelia J., Virani Salim S., Callaway Clifton W., Chang Alexander R., Cheng Susan, Chiuve Stephanie E., Cushman Mary, Delling Francesca N., Deo Rajat, de Ferranti Sarah D., Ferguson Jane F., Fornage Myriam, Gillespie Cathleen, Isasi Carmen R., Jiménez Monik C., Jordan Lori Chaffin, Judd Suzanne E., Lackland Daniel, Lichtman Judith H., Lisabeth Lynda, Liu Simin, Longenecker Chris T., Lutsey Pamela L., Matchar David B., Matsushita Kunihiro, Mussolino Michael E., Nasir Khurram, O’Flaherty Martin, Palaniappan Latha P., Pandey Dilip K., Reeves Mathew J., Ritchey Matthew D., Rodriguez Carlos J., Roth Gregory A., Rosamond Wayne D., Sampson Uchechukwu K.A., Satou Gary M., Shah Svati H., Spartano Nicole L., Tirschwell David L., Tsao Connie W., Voeks Jenifer H., Willey Joshua Z., Wilkins John T., Wu Jason HY., Alger Heather M., Wong Sally S., and Muntner Paul. 2018. “Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association.” Circulation. doi: 10.1161/cir.0000000000000558. - DOI - PubMed
-
- Bonet Javier, and Wood Richard D. 1997. Nonlinear continuum mechanics for finite element analysis: Cambridge university press.

