Methionine restriction prevents onset of type 2 diabetes in NZO mice
- PMID: 30841758
- PMCID: PMC6529347
- DOI: 10.1096/fj.201900150R
Methionine restriction prevents onset of type 2 diabetes in NZO mice
Abstract
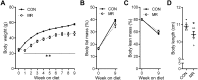
Dietary methionine restriction (MR) is well known to reduce body weight by increasing energy expenditure (EE) and insulin sensitivity. An elevated concentration of circulating fibroblast growth factor 21 (FGF21) has been implicated as a potential underlying mechanism. The aims of our study were to test whether dietary MR in the context of a high-fat regimen protects against type 2 diabetes in mice and to investigate whether vegan and vegetarian diets, which have naturally low methionine levels, modulate circulating FGF21 in humans. New Zealand obese (NZO) mice, a model for polygenic obesity and type 2 diabetes, were placed on isocaloric high-fat diets (protein, 16 kcal%; carbohydrate, 52 kcal%; fat, 32 kcal%) that provided methionine at control (Con; 0.86% methionine) or low levels (0.17%) for 9 wk. Markers of glucose homeostasis and insulin sensitivity were analyzed. Among humans, low methionine intake and circulating FGF21 levels were investigated by comparing a vegan and a vegetarian diet to an omnivore diet and evaluating the effect of a short-term vegetarian diet on FGF21 induction. In comparison with the Con group, MR led to elevated plasma FGF21 levels and prevented the onset of hyperglycemia in NZO mice. MR-fed mice exhibited increased insulin sensitivity, higher plasma adiponectin levels, increased EE, and up-regulated expression of thermogenic genes in subcutaneous white adipose tissue. Food intake and fat mass did not change. Plasma FGF21 levels were markedly higher in vegan humans compared with omnivores, and circulating FGF21 levels increased significantly in omnivores after 4 d on a vegetarian diet. These data suggest that MR induces FGF21 and protects NZO mice from high-fat diet-induced glucose intolerance and type 2 diabetes. The normoglycemic phenotype in vegans and vegetarians may be caused by induced FGF21. MR akin to vegan and vegetarian diets in humans may offer metabolic benefits via increased circulating levels of FGF21 and merits further investigation.-Castaño-Martinez, T., Schumacher, F., Schumacher, S., Kochlik, B., Weber, D., Grune, T., Biemann, R., McCann, A., Abraham, K., Weikert, C., Kleuser, B., Schürmann, A., Laeger, T. Methionine restriction prevents onset of type 2 diabetes in NZO mice.
Keywords: energy expenditure; hyperglycemia; obesity; vegan; vegetarian.
Conflict of interest statement
The authors thank A. Helms, J. Würfel, C. Gumz, and A. Teichmann (German Institute of Human Nutrition) for their skillful technical assistance. The authors thank the staff of the animal housing facility located at the Max Rubner Laboratory (Potsdam-Rehbruecke, Germany) for their skillful assistance and excellent technical support. Linguistic refinements of the text by N. Kühn are gratefully acknowledged. This work was supported by the German Ministry of Education and Research and the Brandenburg State [German Center for Diabetes Research (DZD) Grant 82DZD00302; to A.S.]. T.L. was supported by Grants LA 3042/3-1 and LA 3042/4-1 from the Deutsche Forschungsgemeinschaft (DFG). The authors declare no conflicts of interest.
Figures




References
-
- Laeger T., Albarado D. C., Burke S. J., Trosclair L., Hedgepeth J. W., Berthoud H. R., Gettys T. W., Collier J. J., Münzberg H., Morrison C. D. (2016) Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. 16, 707–716 - PMC - PubMed
-
- Solon-Biet S. M., Cogger V. C., Pulpitel T., Heblinski M., Wahl D., McMahon A. C., Warren A., Durrant-Whyte J., Walters K. A., Krycer J. R., Ponton F., Gokarn R., Wali J. A., Ruohonen K., Conigrave A. D., James D. E., Raubenheimer D., Morrison C. D., Le Couteur D. G., Simpson S. J. (2016) Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 24, 555–565 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

