KAT8 selectively inhibits antiviral immunity by acetylating IRF3
- PMID: 30842237
- PMCID: PMC6446880
- DOI: 10.1084/jem.20181773
KAT8 selectively inhibits antiviral immunity by acetylating IRF3
Erratum in
-
Correction: KAT8 selectively inhibits antiviral immunity by acetylating IRF3.J Exp Med. 2019 Apr 1;216(4):1001. doi: 10.1084/jem.2018177303182019c. Epub 2019 Mar 21. J Exp Med. 2019. PMID: 30898895 Free PMC article. No abstract available.
Abstract
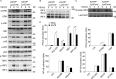
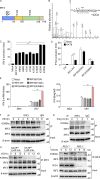
The transcription factor interferon regulatory factor 3 (IRF3) is essential for virus infection-triggered induction of type I interferons (IFN-I) and innate immune responses. IRF3 activity is tightly regulated by conventional posttranslational modifications (PTMs) such as phosphorylation and ubiquitination. Here, we identify an unconventional PTM of IRF3 that directly inhibits its transcriptional activity and attenuates antiviral immune response. We performed an RNA interference screen and found that lysine acetyltransferase 8 (KAT8), which is ubiquitously expressed in immune cells (particularly in macrophages), selectively inhibits RNA and DNA virus-triggered IFN-I production in macrophages and dendritic cells. KAT8 deficiency protects mice from viral challenge by enhancing IFN-I production. Mechanistically, KAT8 directly interacts with IRF3 and mediates IRF3 acetylation at lysine 359 via its MYST domain. KAT8 inhibits IRF3 recruitment to IFN-I gene promoters and decreases the transcriptional activity of IRF3. Our study reveals a critical role for KAT8 and IRF3 lysine acetylation in the suppression of antiviral innate immunity.
© 2019 Huai et al.
Figures





References
-
- Ageta-Ishihara N., Miyata T., Ohshima C., Watanabe M., Sato Y., Hamamura Y., Higashiyama T., Mazitschek R., Bito H., and Kinoshita M.. 2013. Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat. Commun. 4:2532 10.1038/ncomms3532 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

