Environmental control programs the emergence of distinct functional ensembles from unconstrained chemical reactions
- PMID: 30842280
- PMCID: PMC6431231
- DOI: 10.1073/pnas.1813987116
Environmental control programs the emergence of distinct functional ensembles from unconstrained chemical reactions
Abstract
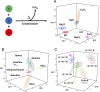
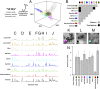
Many approaches to the origin of life focus on how the molecules found in biology might be made in the absence of biological processes, from the simplest plausible starting materials. Another approach could be to view the emergence of the chemistry of biology as process whereby the environment effectively directs "primordial soups" toward structure, function, and genetic systems over time. This does not require the molecules found in biology today to be made initially, and leads to the hypothesis that environment can direct chemical soups toward order, and eventually living systems. Herein, we show how unconstrained condensation reactions can be steered by changes in the reaction environment, such as order of reactant addition, and addition of salts or minerals. Using omics techniques to survey the resulting chemical ensembles we demonstrate there are distinct, significant, and reproducible differences between the product mixtures. Furthermore, we observe that these differences in composition have consequences, manifested in clearly different structural and functional properties. We demonstrate that simple variations in environmental parameters lead to differentiation of distinct chemical ensembles from both amino acid mixtures and a primordial soup model. We show that the synthetic complexity emerging from such unconstrained reactions is not as intractable as often suggested, when viewed through a chemically agnostic lens. An open approach to complexity can generate compositional, structural, and functional diversity from fixed sets of simple starting materials, suggesting that differentiation of chemical ensembles can occur in the wider environment without the need for biological machinery.
Keywords: chemomics; combinatorial chemistry; origin of life; peptides; systems chemistry.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- de Lorenzo V, Galperin M. Microbial systems biology: Bottom up and top down. FEMS Microbiol Rev. 2009;33:1–2. - PubMed
-
- Cronin L. Reaction: A new genesis for origins research? Chem. 2017;2:601–603.
-
- Ashkenasy G, Hermans TM, Otto S, Taylor AF. Systems chemistry. Chem Soc Rev. 2017;46:2543–2554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

