Quantitative Proteomic Analysis Reveals Unfolded-Protein Response Involved in Severe Fever with Thrombocytopenia Syndrome Virus Infection
- PMID: 30842332
- PMCID: PMC6498065
- DOI: 10.1128/JVI.00308-19
Quantitative Proteomic Analysis Reveals Unfolded-Protein Response Involved in Severe Fever with Thrombocytopenia Syndrome Virus Infection
Abstract
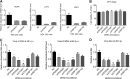
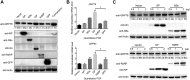
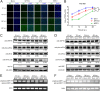
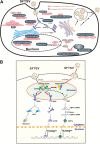
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging, highly pathogenic, infectious disease caused by infection with a newly discovered tick-borne phlebovirus, SFTS virus (SFTSV). Limited information on the molecular mechanism of SFTSV infection and pathogenesis impedes the development of effective vaccines and drugs for SFTS prevention and treatment. In this study, an isobaric tag for relative and absolute quantification (iTRAQ)-based quantitative proteomic analysis of SFTSV-infected HEK 293 cells was performed to explore dynamic host cellular protein responses toward SFTSV infection. A total of 433 of 5,606 host proteins involved in different biological processes were differentially regulated by SFTSV infection. The proteomic results highlighted a potential role of endoplasmic reticular stress-triggered unfolded-protein response (UPR) in SFTSV infection. Further functional studies confirmed that all three major branches of the UPR, including the PKR-like endoplasmic reticulum kinase (PERK), the activating transcription factor-6 (ATF6), and the inositol-requiring protein-1 (IRE1)/X-box-binding protein 1 (XBP1) pathways, were activated by SFTSV. However, only the former two pathways play a crucial role in SFTSV infection. Furthermore, expression of SFTSV glycoprotein (GP) alone was sufficient to stimulate the UPR, whereas suppression of PERK and ATF6 notably decreased GP expression. Interestingly, two other newly discovered phleboviruses, Heartland virus and Guertu virus, also stimulated the UPR, suggesting a common mechanism shared by these genetically related phleboviruses. This study provides a global view to our knowledge on how host cells respond to SFTSV infection and highlights that host cell UPR plays an important role in phlebovirus infection.IMPORTANCE Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne bunyavirus that causes severe fever with thrombocytopenia syndrome in humans, with a mortality rate reaching up to 30% in some outbreaks. There are currently no U.S. Food and Drug Administration-approved vaccines or specific antivirals available against SFTSV. To comprehensively understand the molecular interactions occurring between SFTSV and the host cell, we exploit quantitative proteomic approach to investigate the dynamic host cellular responses to SFTSV infection. The results highlight multiple biological processes being regulated by SFTSV infection. Among these, we focused on exploration of the mechanism of how SFTSV infection stimulates the host cell's unfolded-protein response (UPR) and identified the UPR as a common feature shared by SFTSV-related new emerging phleboviruses. This study, for the first time to our knowledge, provides a global map for host cellular responses to SFTSV infection and highlighted potential host targets for further research.
Keywords: ATF6; ER stress; PERK; SFTSV; XBP1; quantitative proteomics; unfolded-protein response; virus-host interaction.
Copyright © 2019 Zhang et al.
Figures




References
-
- Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364:1523–1532. doi:10.1056/NEJMoa1010095. - DOI - PMC - PubMed
-
- Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, Senba T, Kaneyuki S, Sakaguchi S, Satoh A, Hosokawa T, Kawabe Y, Kurihara S, Izumikawa K, Kohno S, Azuma T, Suemori K, Yasukawa M, Mizutani T, Omatsu T, Katayama Y, Miyahara M, Ijuin M, Doi K, Okuda M, Umeki K, Saito T, Fukushima K, Nakajima K, Yoshikawa T, Tani H, Fukushi S, Fukuma A, Ogata M, Shimojima M, Nakajima N, Nagata N, Katano H, Fukumoto H, Sato Y, Hasegawa H, Yamagishi T, Oishi K, Kurane I, Morikawa S, Saijo M. 2014. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis 209:816–827. doi:10.1093/infdis/jit603. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

