Autophagy induction via STING trafficking is a primordial function of the cGAS pathway
- PMID: 30842662
- PMCID: PMC9417302
- DOI: 10.1038/s41586-019-1006-9
Autophagy induction via STING trafficking is a primordial function of the cGAS pathway
Abstract
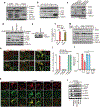
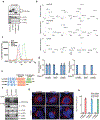
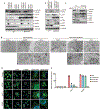
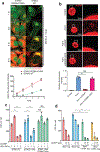
Cyclic GMP-AMP (cGAMP) synthase (cGAS) detects infections or tissue damage by binding to microbial or self DNA in the cytoplasm1. Upon binding DNA, cGAS produces cGAMP that binds to and activates the adaptor protein STING, which then activates the kinases IKK and TBK1 to induce interferons and other cytokines2-6. Here we report that STING also activates autophagy through a mechanism that is independent of TBK1 activation and interferon induction. Upon binding cGAMP, STING translocates to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) and the Golgi in a process that is dependent on the COP-II complex and ARF GTPases. STING-containing ERGIC serves as a membrane source for LC3 lipidation, which is a key step in autophagosome biogenesis. cGAMP induced LC3 lipidation through a pathway that is dependent on WIPI2 and ATG5 but independent of the ULK and VPS34-beclin kinase complexes. Furthermore, we show that cGAMP-induced autophagy is important for the clearance of DNA and viruses in the cytosol. Interestingly, STING from the sea anemone Nematostella vectensis induces autophagy but not interferons in response to stimulation by cGAMP, which suggests that induction of autophagy is a primordial function of the cGAS-STING pathway.
Conflict of interest statement
The authors declare no competing financial interests.
Figures










Comment in
-
Autophagy induced by STING, an unnoticed and primordial function of cGAS.Cell Mol Immunol. 2019 Aug;16(8):683-684. doi: 10.1038/s41423-019-0240-2. Epub 2019 May 29. Cell Mol Immunol. 2019. PMID: 31142798 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

